Conventional vaccine adjuvants primarily rely on molecular binding and biochemical stimulation to activate immune responses, which often leads to limited efficacy in elderly or low-responsive populations. How to introduce physical regulation into immune activation remains an open challenge.
A research team led by Professor XIA Yufei at the Institute of Process Engineering (IPE), Chinese Academy of Sciences, has demonstrated that redesigning aluminum adjuvants into a deformable three-dimensional mechanical interface can significantly enhance immune activation. By constructing aluminum-stabilized Pickering emulsions (ASPEs), the team enabled dendritic cells (DCs) to actively sense interfacial mechanical cues, thereby amplifying immune responses.
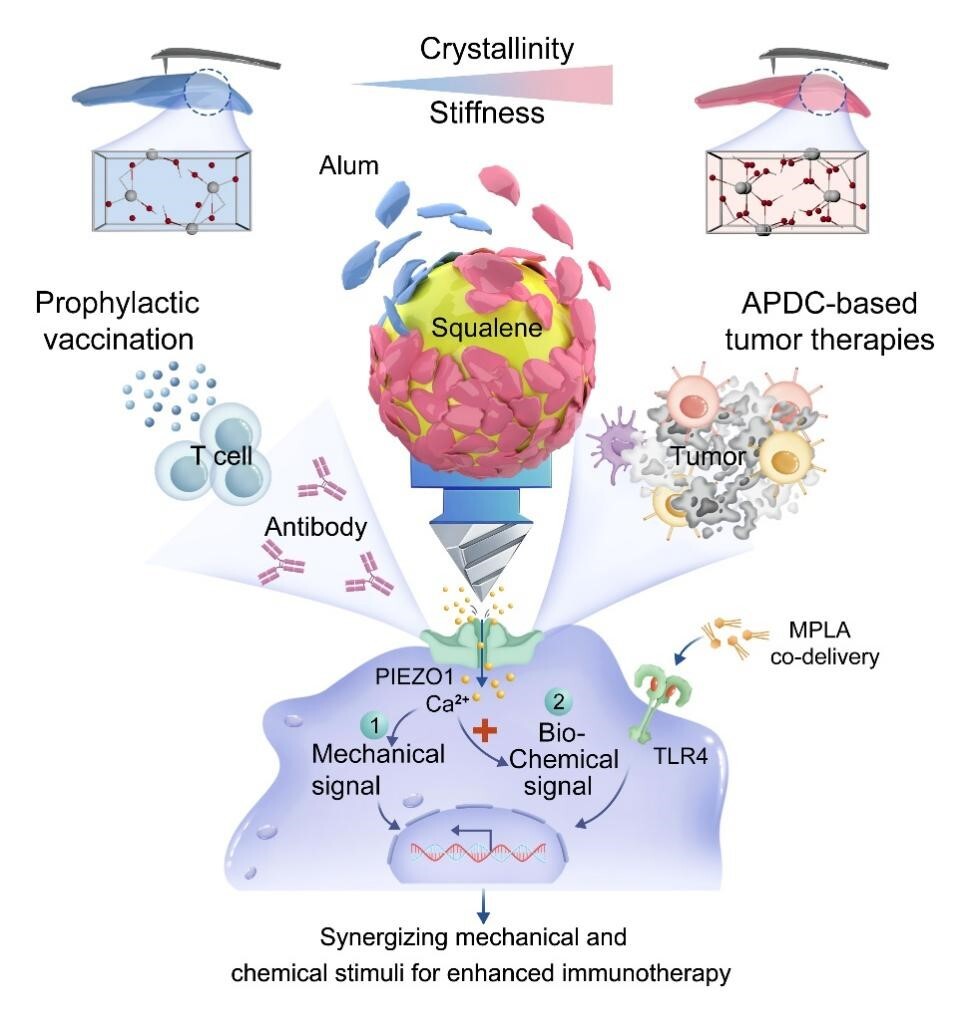
Figure 1 Interfacial mechano-biochemical dual signaling potentiates immune activation by Aluminum-stabilized Pickering emulsions (ASPEs)
Unlike conventional particulate adjuvants with limited membrane contact, ASPE droplets deform upon contact with DC membranes, increasing interfacial contact area and delivering controllable mechanical stress. By tuning the crystallinity of aluminum nanoparticles, the interfacial stiffness can be precisely regulated, allowing graded mechanical stimulation. Stronger mechanical cues directly activate the mechanosensitive ion channel PIEZO1, trigger Ca²⁺ influx, and promote antigen cross-presentation.
When combined with the TLR4 agonist monophosphoryl lipid A (MPLA), the ASPE platform achieves synergistic mechano-biochemical activation. Compared with the clinically used Alum+MPLA formulation, ASPE-M induces stronger DC maturation, enhanced Th1-biased immunity, and robust CD8⁺ T-cell responses. Notably, these effects remain pronounced in aged mouse models and significantly improve therapeutic outcomes in dendritic cell-based melanoma immunotherapy, especially when combined with PD-1 blockade.
This work establishes interfacial mechanics as a critical and programmable dimension of immune regulation, complementary to traditional biochemical signaling. The findings provide a new strategy for vaccine and immunotherapy design, with particular promise for improving immune efficacy in aging and immunocompromised populations.
The study was published in Cell Biomaterials on December 10 (DOI: 10.1016/j.celbio.2025.100281).
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号