A team of researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and Nanjing Normal University has recently reported a phosphidation strategy for preparing a substitutional solid solution composed of platinum (Pt) and phosphorus (P) elements. Their efforts would inspire more scientific ideas for the design of high-efficiency and cost-effective materials for various applications.
The study was published at Small (DOI: 10.1002/smll.202409927).
Typically, the significant disparities in atomic size, crystal structure, electronegativity, and bonding modes between metals and nonmetals, alongside their low mutual solubility, hinder the formation of substitutional solid solutions. Instead, interstitial solid solutions are more common, where solute atoms, or nonmetal atoms, are embedded in the interstitial positions formed by the stacking of solvent or host atoms (metal atoms).
In contrast, the Pt-P system presents a unique perspective in fundamental materials science. The formation of a substitutional solid solution between Pt and P does not violate the Hume-Rothery rules in terms of crystal structure, electronegativity, and atomic size. Particularly, the relative atomic size difference between Pt and P atoms is approximately 19.1%, suggesting the potential for a limited substitutional solid solution.
IPE researchers found that when using tri-n-octylphosphine (TOP) to phosphide the preformed Pt nanoparticles, P atoms are able to replace some of the Pt atoms located at their lattice sites, rather than entering the tetrahedral or octahedral interstitial positions of the face-centered Pt crystal, thus enabling the formation of a Pt-P substitutional solid solution.
The researchers first prepared worm-like Pt nanoparticles, and then phosphorylated them with TOP at high temperatures. Transmission electron microscope observations revealed that this phosphorylation induced significant changes in particle morphology, transforming the worm-like particles into spherical ones.
The formation of the substitutional solid solution was collectively confirmed through various characterization techniques.
Theoretical calculations indicate that a stable face-centered cubic structure can accommodate up to approximately 10% P doping, aligning well with experimental observations and predictions based on atomic size differences.
The limited doping of P can also be elucidated through fundamental materials science theories. While P doping increases entropy within the system, lower temperatures yield a predominance of changes in internal energy. Consequently, there exists an upper limit for P doping in Pt that maintains the stability of the Pt-Pt solid solution by minimizing Gibbs free energy. The consistency between experimental findings and theoretical calculations underscores the reliability of the results.
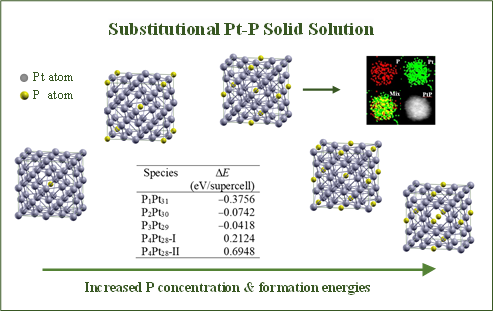
Fig. 1. Formation energies and illustrations showing the relaxed cubic Pt-P substitutional solid solutions. (Image by YANG Jun)
“These insightful findings in Pt phosphidating process will not only deepen our understanding of materials science but also enrich the contents of fundamentals of materials science and engineering textbooks,” said Prof. YANG Jun from IPE, the corresponding author of the study.
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search



 京公网安备110402500047号
京公网安备110402500047号