Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have recently developed a novel lysosomal strategy for clearing viruses and their variants. This lysosomal “TRAP” (lysoTRAP) shows potential for inhibiting infection by viruses and variants in cell, mouse, hamster, and human lung organoid models.
Results of the study were published in Nature Communications (DOI:10.1038/s41467-024-54505-6).
Viral infections pose a serious threat to human health and safety. The binding of viral ligands to host cell receptors is essential for virus entry. Numerous studies have explored the development of medical agents (such as antibodies and small molecule inhibitors) to prevent this binding.
The effectiveness of these agents relies on their specificity to the viral ligands. Therefore, these strategies can be rendered ineffective by viral mutation, which is now known to occur frequently and poses a constant threat to public health.
LysoTRAP was inspired by the idea of mimicking viral entry to host cells and macrophage-mediated viral clearance. The IPE researchers used severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a testbed and initially modified the surface of lysosomes with the viral protein receptor angiotensin-converting enzyme 2 (ACE2) as “bait”. In this way, the researchers constructed lysoTRAP to selectively capture, internalize, and degrade SARS-CoV-2.
“LysoTRAP is a pioneer in exploring lysosomes as a therapeutic and represents a novel anti-virus strategy,” said Prof. MA Guanghui from IPE.
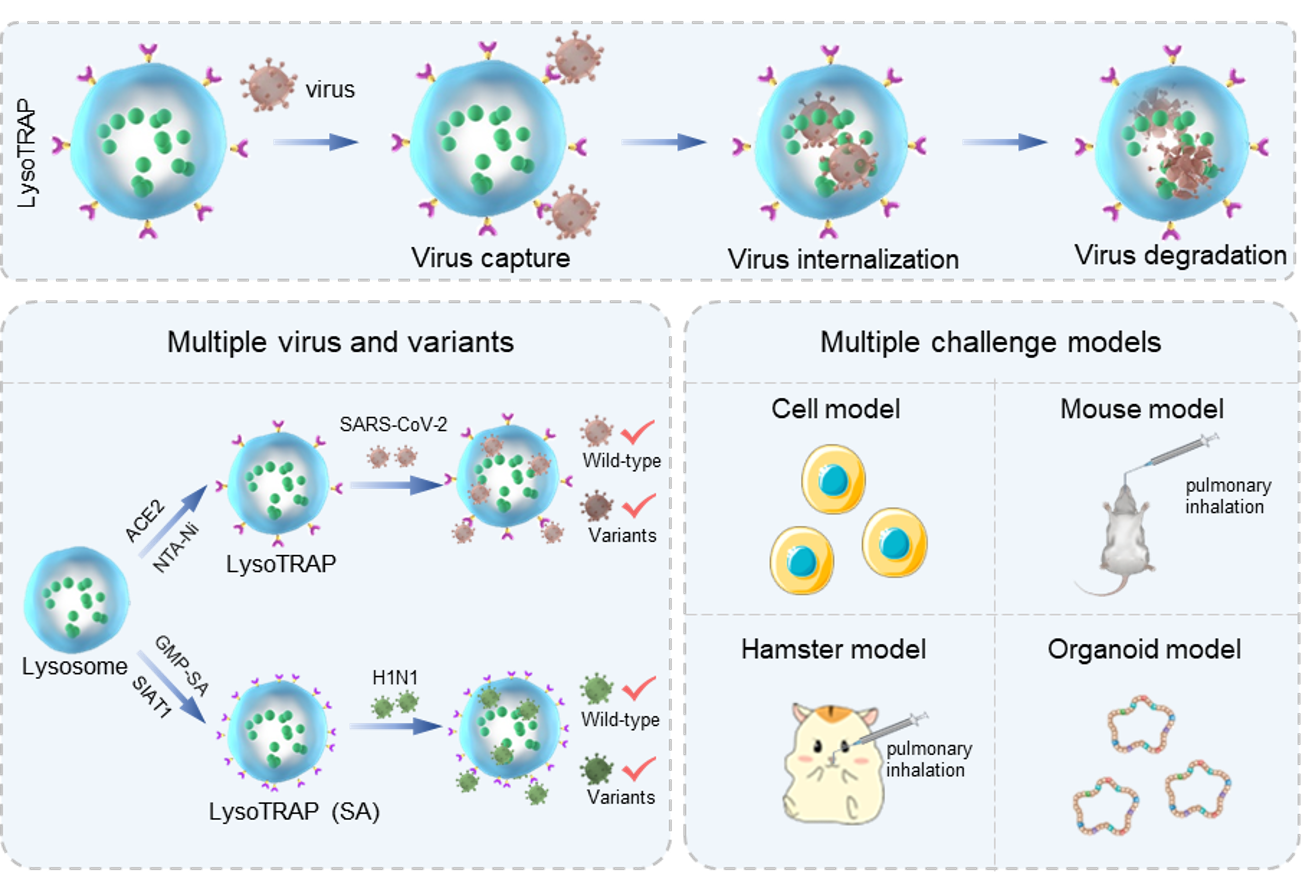
Figure 1. Schematic for showing lysoTRAP programmed performance in capturing, internalizing, and degrading multiple viruses and variants for virus clearance in multiple virus challenge models (Image by WEI Wei).
Prof. MA explained that lysoTRAP exploited the spike/ACE2 affinity to capture SARS-CoV-2 and internalized the virus to completely isolate it from the host cell. It then used lysosomal hydrolases to degrade the virus to profoundly reduce the likelihood of viral escape or continued infectivity.
In the cell model, lysoTRAP efficiently inhibited the infection of wild-type pseudotyped SARS-CoV-2 and nine variants. In the hamster model, it cleared both authentic wild-type SARS-CoV-2 and the Omicron variant, thus alleviating viral pneumonia.
To approximate the genetic and epigenetic features of human lung, the researchers established a human lung organoid model and found that lysoTRAP outperformed the current standard of care (EIDD-2801 [riboside analogs] and RBD antibodies) against authentic wild-type SARS-CoV-2. Working with the Omicron variant, the inhibitory effect of both free EIDD-2801 and RBD antibodies showed an obvious decrease, but lysoTRAP didn’t.
Distinct from other therapeutic agents which always targeted viral ligands, lysoTRAP was not vulnerable to loss of efficacy from viral mutations.
“Given that the working principle for lysoTRAP’s recognition of SARS-CoV-2 is the ACE2 protein, any infective variants that bind ACE2 are sensitive to lysoTRAP-mediated clearance,” said Prof. WEI Wei from IPE.
Extending beyond SARS-CoV-2, the researchers replaced the ACE2 protein receptor with the sialic acid (SA) saccharide receptor and again found potent clearance of influenza A by lysoTRAP in cell, mouse, and human lung organoid models, demonstrating the flexibility of the “TRAP” system.
“The proposed lysoTRAP platform holds significant promise for therapeutic development, offering a unique strategy to clear viral infections by leveraging the natural degradative functions of lysosomes,” said a peer reviewer from Nature Communications, who noted that it introduced “a very innovative approach for combating viral infections.”
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search



 京公网安备110402500047号
京公网安备110402500047号