Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have developed a probiotic-based therapeutic that synergistically restores intestinal redox and microbiota homeostasis. This therapeutic effectively relieved inflammation and reduced colonic damage in mouse and non-human primate (NHP) models of colitis. The study was published in Cell Host & Microbe (DOI: 10.1016/j.chom.2024.07.028).
Probiotics are potential treatments for ulcerative colitis (UC), but their efficacy is frequently compromised by gastrointestinal conditions that limit adhesion and activity. Moreover, the complicated pathological environment of inflamed tissue also implies the presence of other pathogenic factors.
To address these issues, researchers, employing machine learning and bioinformatics analyses, identified the Lactobacillus genus as a suitable candidate for modulating the gut microbiota, and pinpointed oxidative stress as a pivotal pathogenic factor for targeted intervention.
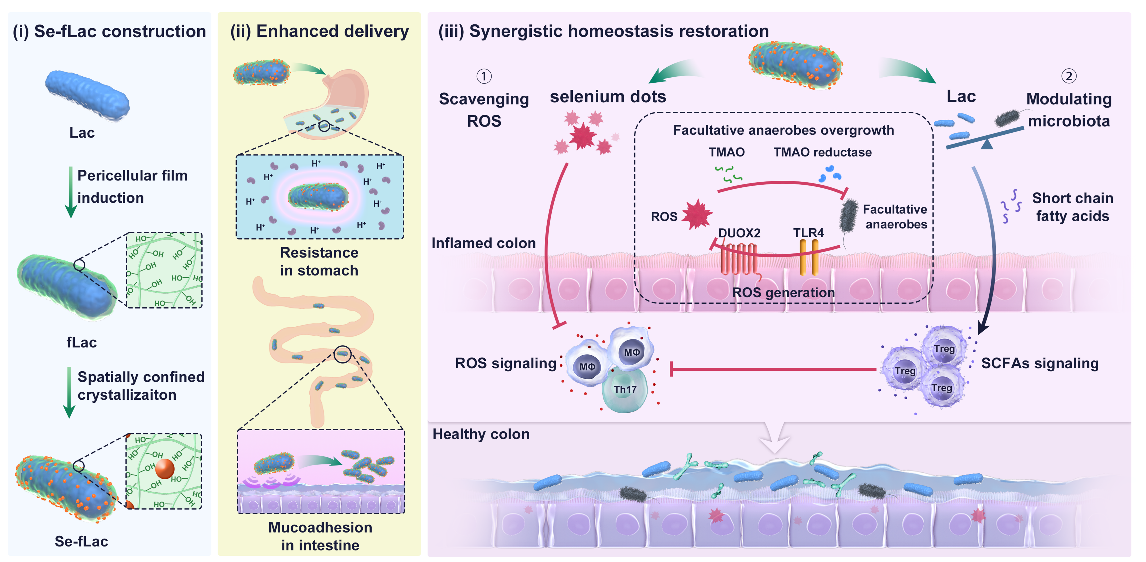
Fig. Schematic illustration of the preparation, enhanced oral delivery, and synergistic homeostasis restoration of Se-fLac. (Image by WEI Wei)
For verification, researchers collected and analyzed fecal samples from a cohort of healthy donors and UC patients. The results showed a decreased prevalence of Lactobacillus and increased oxidative stress in UC patients.
“Further considering inflammation severity, we discovered correlations between any two aspects of Lactobacillus abundance, 8-hydroxy-2-deoxyguanosine levels, and C-reactive protein values in UC patients, which revealed the close association of these two aspects in UC exacerbation,” said Prof. CUI Yimin from Peking University First Hospital, one of the corresponding authors.
These findings indicated that, beyond delivering adequate viable Lactobacillus, endowing Lactobacillus with the function of scavenging reactive oxygen species (ROS) could further improve UC treatment. Considering that selenium is a nutritional trace element conferring antioxidant effects, researchers proposed that the codelivery of selenium and Lactobacillus could fulfill the synergistic restoration of intestinal redox-microbiota homeostasis.
“We developed a new method to in situ grow selenium dots which are embedded in the pericellular film surrounding the Lactobacillus casei (Lac) cell wall. The resulting Se-fLac not only endowed Lac cells with ultrahigh ROS scavenging activity, but also enhanced gastric acid resistance and intestinal mucoadhesion of Lac cells after oral administration,” said Prof. MA Guanghui from IPE.
At the lesion site, Se-fLac prominently scavenged ROS and had the tendency to modulate gut microbiota, and these two aspects were further observed with a potentially mutual benefit.
“To validate the therapeutic efficacy of Se-fLac, we compared it with 5-aminosalicylic acid treatment, VSL#3 supplements, and their combination. Se-fLac significantly outperformed these three counterparts in all therapeutic indices,” said Prof. WEI Wei from IPE.
Although the UC mouse models have been widely utilized to examine therapeutic efficacies, these mouse models might fail to accurately simulate the anatomic structures and physiological functions of the gastrointestinal tract, as well as pathological features of UC in humans.
“This motivated us to establish an NHP model to bridge mouse- and human-based investigations of Se-fLac, and the potent therapeutic efficacies highlighted the strong translational potential of Se-fLac to develop clinically relevant UC treatments,” said Prof. WEI.
“This manuscript is an excellent addition to the field,” said one peer reviewer from Cell Host & Microbe.
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号