A research team led by Prof. GAO Xiaodong from the Institute of Process Engineering of the Chinese Academy of Sciences has developed an in vitro detection method for the translocation of M5GN2-PP-Dol (an N-glycosylated glycolipid intermediate), and has demonstrated that Rft1 protein catalyze the transmembrane lipid bilayer translocation of M5GN2-PP-Dol.
The study was published in Nature Communications on June 17th (DOI: 10.1038/s41467-024-48999-3).
N-linked glycosylation is an essential protein post-translational modification common to all three domains of life (Archaeas, Eukaryotes and Prokaryotes), which plays a crucial role in the structure and function of glycoproteins.
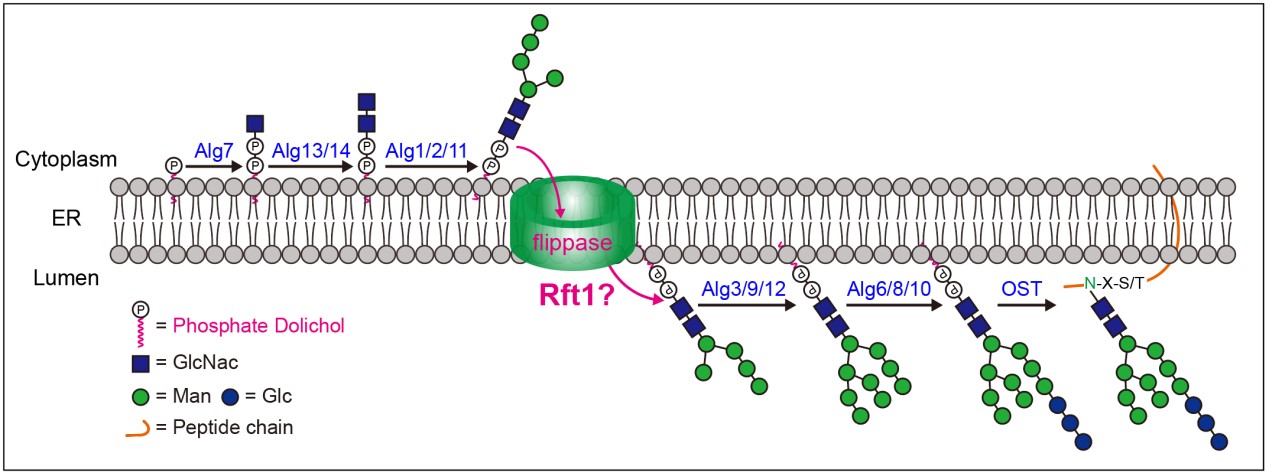
Fig.1 The assembly of lipid-linked oligosaccharide on the Endoplasmic Reticulum (Image by CHEN Shuai)
The N-glycosylation modification process in eukaryotic cells begins with the assembly of dolichol-linked oligosaccharide precursors on the endoplasmic reticulum (ER) membrane. The N-glycosylated glycolipid intermediate M5GN2-PP-Dol would translocate across the ER membrane into the lumen for subsequent assembly (Fig. 1). However, the question of which protein (flippase) mediates the translocation of M5GN2-PP-Dol has been debated for over two decades.
To address this question, it is necessary to develop a more suitable flippase activity detection system. In this study, the research team developed an in vitro flippase activity assay system based on mannosidase-coupled ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) for quantitative detection.
This flippase activity assay system was a completely reconstituted in vitro assay for M5GN2-PP-Dol translocation (Fig. 2). In these liposomes, M5GN2-PP-Dol adopted a random distribution between the inner and outer membrane leaflets, where about half of the M5GN2-PP-Dol faces the lumen while the other half faces outside. M5GN2-PP-Dol in the outer leaflet of liposomes was digested to M3GN2-PP-Dol by α1-2 mannosidase.
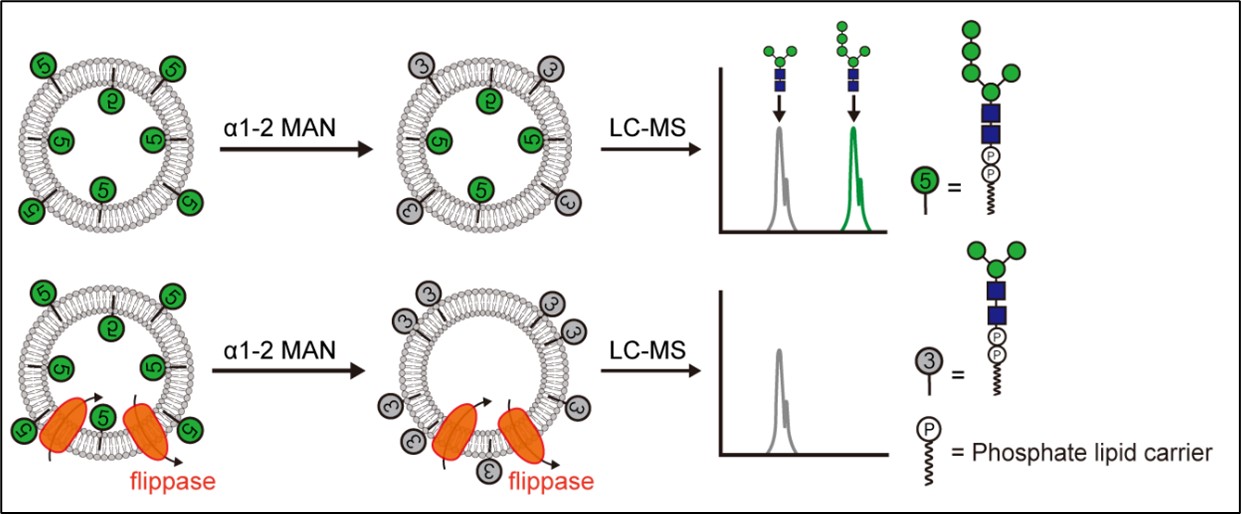
Fig.2 Schematic of α1-2 mannosidase-based translocation assay by UPLC-MS analysis (Image by CHEN Shuai)
In the absence of a flippase, there was a 1:1 distribution of M3GN2:M5GN2. Flippase activity was measured as an increase in this ratio. With the addition of flippase to these liposomes, the liposomes would catalyze the exchange of M5GN2-PP-Dol between leaflets of liposomes, and therefore cause a greater conversion of M5GN2 to M3GN2 (up to 100%) due to the concomitant increased exposure of M5GN2-PP-Dol to α1-2 mannosidase.
In the detection system, liposomes containing only purified Rft1 and M5GN2-PP-Dol was constructed in vitro to exclude interference from other proteins. The quantitative detection results of UPLC-MS demonstrate that purified Rft1 catalyzes the translocation of M5GN2-PP-Dol across the lipid bilayer. The subsequent experiments demonstrated that liposomes reconstituted with a membrane fraction without Rft1 displayed no flippase activity in vitro. These data confirm the molecular identity of Rft1 as the M5GN2-PP-Dol ER flippase.
A peer reviewer from Nature Communications called the research "rigorous and of high quality" and said that "The manuscript is clear, concise and well-structured. The conclusions are strongly supported by the data presented."
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail:xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号