The development of a universal influenza vaccine is crucial for tackling the mutating strains of influenza viruses.
Researchers from the Institute of Process Engineering of Chinese Academy of Sciences have constructed a novel vaccine using cationic solid lipid nanoparticles as adjuvants combined with universal influenza antigen to form NM2e@DDAB/PLA nano-vaccine. At present, the efficacy and safety of the vaccine in preventing influenza virus have been confirmed in mouse animal models.
The study was published on May 9 in ACS Nano (DOI: 10.1021/acsnano.4c00668).
One of the main approaches in the current development of a universal influenza vaccine involves targeted design against highly conserved viral protein regions across different virus subtypes, with key targets including Hemagglutinin (HA), Neuraminidase (NA), the extracellular domain of M2 protein (M2e), and Nucleoprotein (NP). Due to the inherent high variability of HA and NA antigens, M2e and NP antigens have become the breakthrough points for research.
However, studies have shown that the combination of the adjuvant aluminum hydroxide (Al(OH)3) with the recombinant NP-M2e fusion protein (NM2e) antigen, expressed in E. coli, has proven challenging in inducing potent secretion of interferon-gamma (IFN-γ) and activation of cytotoxic T lymphocytes (CTLs).
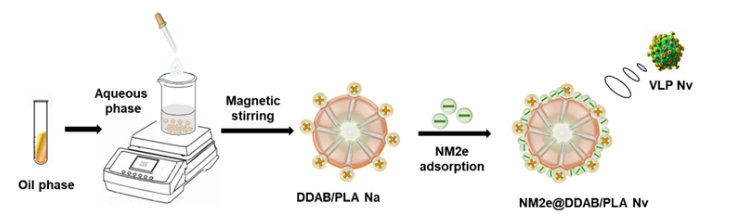
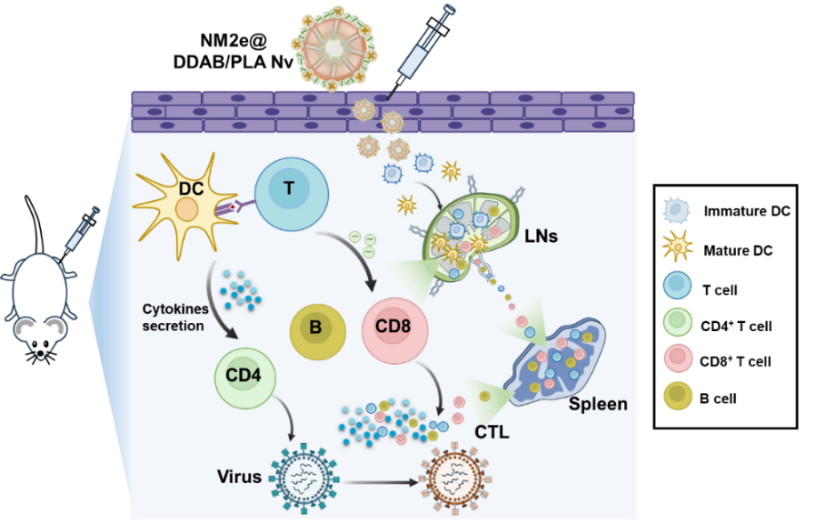
Figure Construction of NM2e@DDAB/ PLA nano-adjuvant vaccine and schematic diagram of its action mode after immunization (Image by GAO Yuan)
"To address the limitations of current aluminum salt adjuvanted vaccines, we engineered a novel polymer-lipid nanoparticle adjuvant loaded with the NM2e antigen, designed to serve as a universal influenza vaccine.” said Prof. WANG Lianyan, the corresponding author of this study.
This vaccine capitalizes on its small particle size and positive surface charge to significantly enhance antigen-presenting cells uptake, facilitating antigen escape into the cytosol and augmenting cross-presentation of the antigen. In addition, it not only effectively promotes the activation and maturation of dendritic cells in vivo, supporting their residence in lymph nodes, but also efficiently stimulates IFN-γ production and CTL responses in secondary lymphoid organs.
Collaborating with Prof. TAN Wenjie's team, researchers discovered that mice immunized with this vaccine displayed robust cross-protective capabilities against homo-and heterosubtype influenza virus challenges post-immunization.
Meanwhile, the safety of NM2e@DDAB/PLA nano-vaccine was verified by serum biochemical analysis and pathological tissue section experiments.
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail:xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号