Thanks to the low cost of raw materials and long lifespan rendered by their stable structure, iron-based phosphate cathodes are regarded as one of the most promising cathodes for Na-ion batteries (NIBs).
Among various iron-based phosphate cathodes, the maricite phase of NaFePO? (NFP) is usually believed to be electrochemically inert due to the absence of effective Na+ ion diffusion channels, whereas Na2FeP2O7 shows open three-dimensional Na+ channels but low theoretical capacity with air instability. In contrast, Na4Fe3(PO4)2P2O7 (NFPP-4.0 ) allows a high theoretical capacity of ~130 mA h g-1 and 3.1 V-level output as well as good air stability. Therefore, NFPP-4.0 has attracted great attention in recent years from researchers and those interested in industrial application.
However, the practical reversible capacity of NFPP-4.0 is restricted to the 110-120 mA h g-1 range due to structural defects, which is far lower than its theoretical value. As a result, further improving the reversible capacity and energy density of iron-based phosphates has been a challenge.
In response to this problem, a research group led by Prof. ZHAO Junmei from the Institute of Process Engineering of the Chinese Academy of Sciences recently reported an effective strategy to improve the reversible capacity and energy density of iron-based phosphate cathodes through the activation of the inert NFP phase. The study was published in the Journal of the American Chemical Society on March 28 (DOI: 10.1021/jacs.3c14452).
In order to increase their theoretical capacity and energy density, the researchers proposed incorporation of the maricite NFP phase into the NFPP-4.0 host to create iron-based phosphates with more active Fe2+ contents based on the formation of heterogeneous structure. The final optimized Na4.5Fe3.5(PO4)2.5(P2O7) cathode (i.e., NFPP-4.5; the mole ratio of NFP/NFPP-4.0 is 0.5:1) enables full activation of the inert NFP and delivers a reversible capacity of over 130 mA h g-1, thereby increasing the energy density to 400 W h kg-1, which is close to the highest energy density of iron-based polyanionic cathodes used in NIBs.
X-Ray diffraction (XRD) revealed that NFPP-4.5 is composed of a bi-phase intergrowth heterogeneous structure based on the NFP and NFPP-4.0 phases, in which the NFP nanodomains are distributed in the continuous NFPP-4.0 matrix, thus generating compatible and percolating interfaces. Such two-phase interfaces can trigger Na+ transportation in the NFP nanodomains, contributing to its electrochemical activation in the composite cathodes.
In situ XRD also confirmed that NFP undergoes amorphization transition upon its first charging and maintains this amorphous nature in following cycles, which is key to achieving the high capacity of NFPP-4.5 with the activation of inert NFP.
The practical application of NFPP-4.5 was validated by the scale-up synthesis of kg-level products and pouch cells, revealing desirable fast-charge capability and superior cycling performance. When charged at 5 C (i.e., five times the battery charging capacity, implying a relatively high charging current), the reversible capacities of the pouch cells still maintained 80% retention compared to 0.1 C (i.e., 0.1 times the battery charging capacity). The pouch cells demonstrated an excellent capacity retention of over 88% after cycling at 3 C (i.e., three times the battery charging capacity) 2000 times, demonstrating huge application prospects.
All in all, this work provides guidance on developing low-cost, high-performance polyanion cathodes that can be used in NIBs.
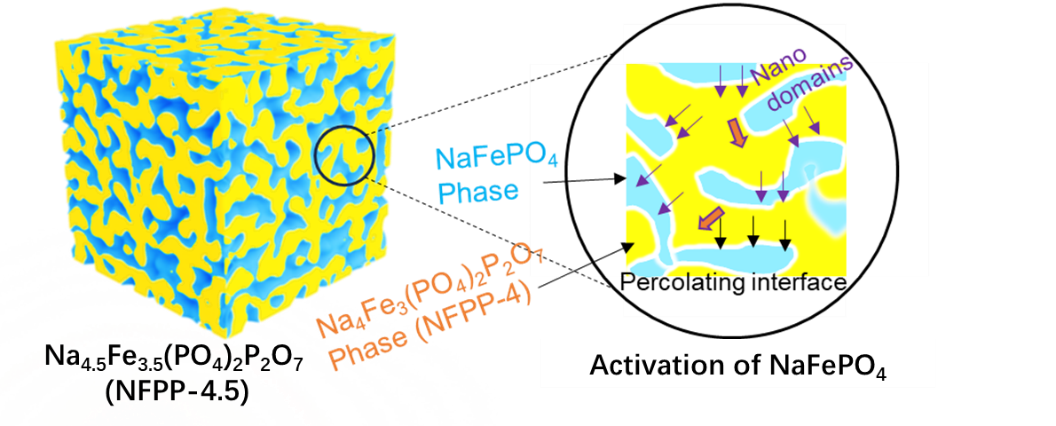
Fig. 1 Bi-phase intergrowth heterogeneous structure formed by NFP and NFPP-4.0 in the new composite material NFPP-4.5 and the activation of NFP (Image by XU Chunliu)
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail:xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号