A research team led by Prof. ZHU Qingshan from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences investigated the electronic structure transition from NiAs- to MnP- type CoPxS1-x compounds and how the transition influences catalytic activity of polysulfide conversion reactions in lithium-sulfur batteries.
By tuning the coordination structure of active centers and optimizing the d-orbitals, they are able to design the catalysts more rationally.
The study was published in ACS Nano on Jan. 30 ( DOI: 10.1021/acsnano.2c12436).
Lithium-sulfur batteries are promising energy storage systems due to their high theoretic capacity (1675 mAh g-1) and specific energy (2600 Wh kg-1). However, polysulfide shuttling leads to the decay of batteries, limiting the practical applications.
Catalysts can accelerate the conversion of polysulfides and relatively suppress the shuttling, which serves as the effective approach to improving cyclability of Li-S batteries. The lack of understanding of the catalytic mechanism and origin poses an obstacle to the design of Li-S catalysts.
Most studies on catalysts reply on the trials and errors and lack the theoretic understanding and rational design. Hexagonal NiAs- and orthogonal MnP-type compounds are most common AB compounds for Li-S batteries.
"Compared with NiAs, cations in MnP-type compounds shift along the plane vertical to c-axis, leading to the shortened inter-center distance of two-edge polyhedra and enhancing the metal-metal bonding," said ZHANG Huigang, a principal investigator at IPE. Their electronic structures change with the coordinated polyhedra. The resultant upshift or downshift of d-band would determine the bonding of catalysts and polysulfides.
Based on the transition, the researchers doped CoP with sulfur and distorted the [CoP6] polyhedra to shift the cation centers. As a result, the dz2 orbital shifted down and the dxz/dyz orbital moved up, enhancing the bonding toward polysulfides.
To realize practical applications of high-loading Li-S batteries, they electrospun CoPxS1-x inside porous carbon nanofibers, forming chain-like nanoreactors, which confined and effectively catalyzed the polysulfide conversion reaction.
The catalyst design and nanofiber construction enable excellent cyclability of high-loading Li-S batteries.
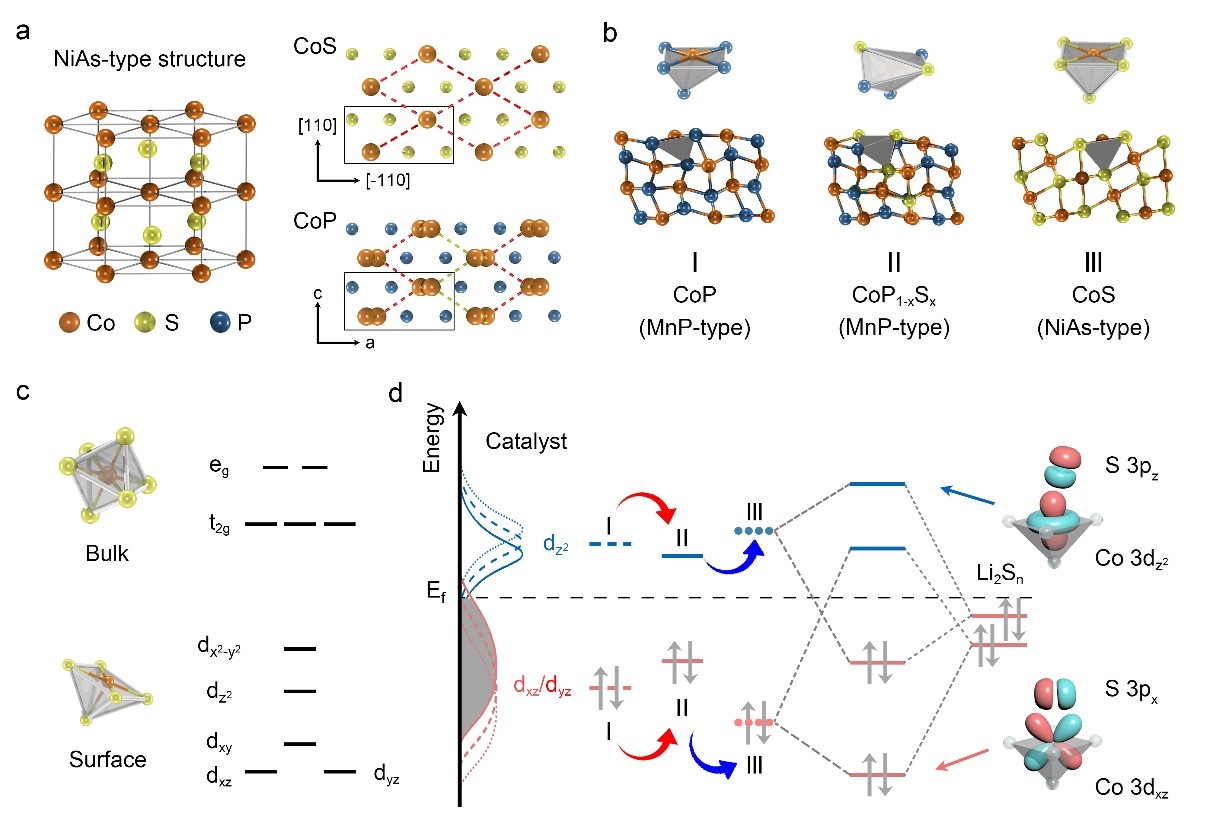
Tuning catalytic activity by modulating the bonding between catalyst and polysulfides on the basis of the NiAs- and MnP-type transition of AB compounds (Image by ZHANG Huigang)
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail:xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号