Na-ion batteries are promising large-scale energy storage technology owing to the abundant raw material resources, low cost and high safety. Sodium vanadium fluorophosphate (Na3(VOPO4)2F) with theoretical energy density of 480 Wh/Kg is regarded as a strong candidate among various cathode materials. However, the intrinsic low conductivity and high energy-consumption during synthesis process hinder its commercialization.
Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and Institute of Physics of the Chinese Academy of Sciences developed the “one-step mechanochemical method” to rapidly prepare the polyanionic compound sodium vanadium fluorophosphate as the cathode materials for Na-ion batteries, which exhibited excellent rate performance and cycle stability.
This work was published in Nature Communications on May 14 (DOI: 10.1038/s41467-021-23132-w).
The green synthesis with low cost and high production efficiency is a great challenge to commercialize the sodium vanadium fluorophosphate.
The prepared Na3(VOPO4)2F/KB composite delivered a high discharge capacity of 142 mAh g-1 at 0.1C. The extra capacity beyond the theoretical specific capacity (130 mAh g-1) benefited from the interfacial charge storage. Moreover, a specific capacity of 112 mAh g-1 can be obtained even at 20 C, which means this Na-ion battery could be fully charged/discharged in 3 minutes. Superior cycle stability of this composite was demonstrated by an ultrahigh cycling stability with 98% retention over 10,000 cycles. Commercial grade 26650 cylindrical battery assembled by the kg level amplification product manifested its potential for industrialization.
“High resolution transmission electron microscopy revealed that the nanocrystallines of Na3(VOPO4)2F about 30 nm were embedded in the carbon framework, which facilitated the rapid conduction of electrons and Na ions. The reversible structural evolution and negligible volume change of Na3(VOPO4)2F/KB composite during charging/discharging were also demonstrated by in situ X-ray diffraction and 23Na nuclear magnetic resonance spectrum.” said by Prof. ZHAO Junmei.
This mechanochemical method could shorten the 7-days production time of liquid phase method to 30 minutes and guarantees the production efficiency for industrial applications.
“It also provides a feasible strategy to improve the rate performance and cycle performance of cathode materials. Besides, the kilogram-scale product indicates the mechanochemical method is suitable for rapid large-scale production electrode materials for Na-ion batteries.” said Prof. ZHAO Junmei, a co-corresponding author of the paper.
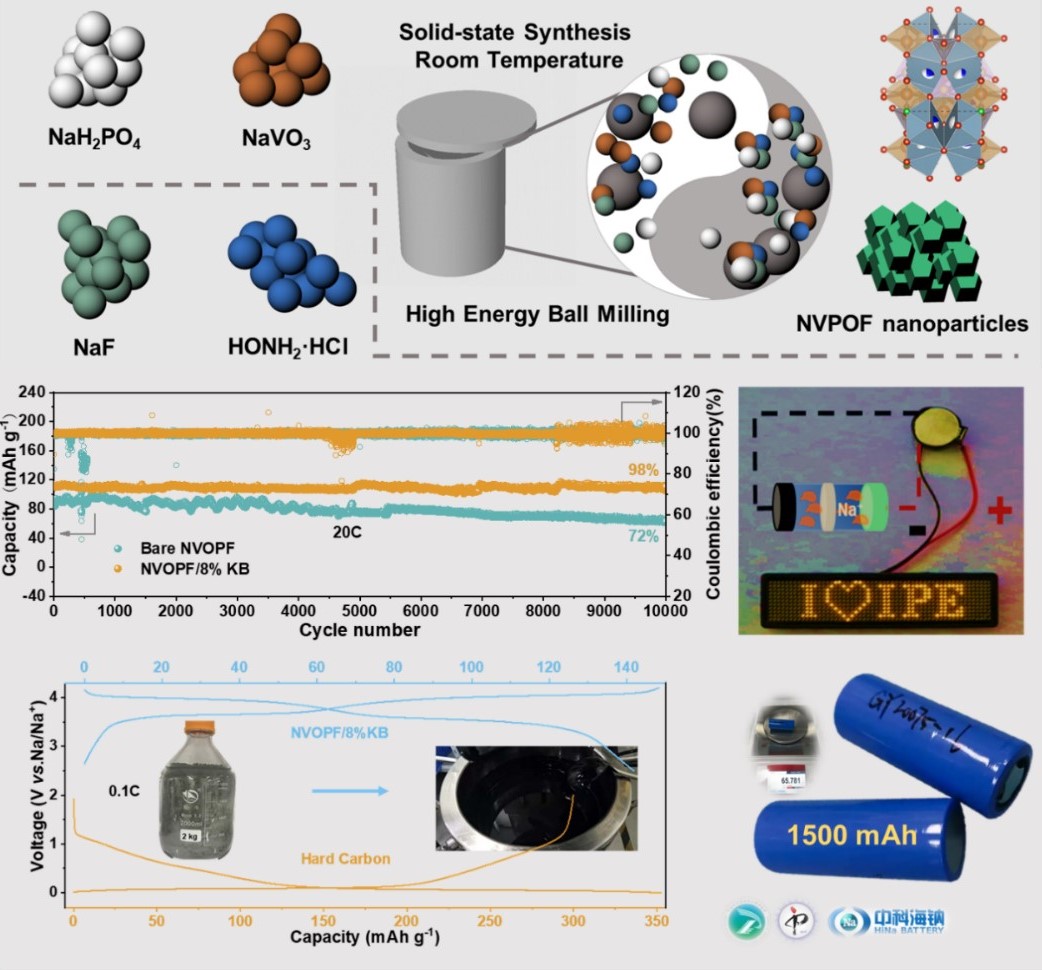
The electrochemical performance of sodium vanadium fluorophosphate synthesized by mechanochemical method.
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号