Micropollutants such as endocrine disrupters, pesticides, and pharmaceuticals have detrimental effects on public health and aquatic ecosystems even at trace level. Biocatalytic membrane exhibits high micropollutants removal efficiency due to the integration of enzyme catalysis and membrane separation.
Achieving long-term stability and high catalytic efficiency at the same time remains challenging in biocatalytic membrane fabrication.
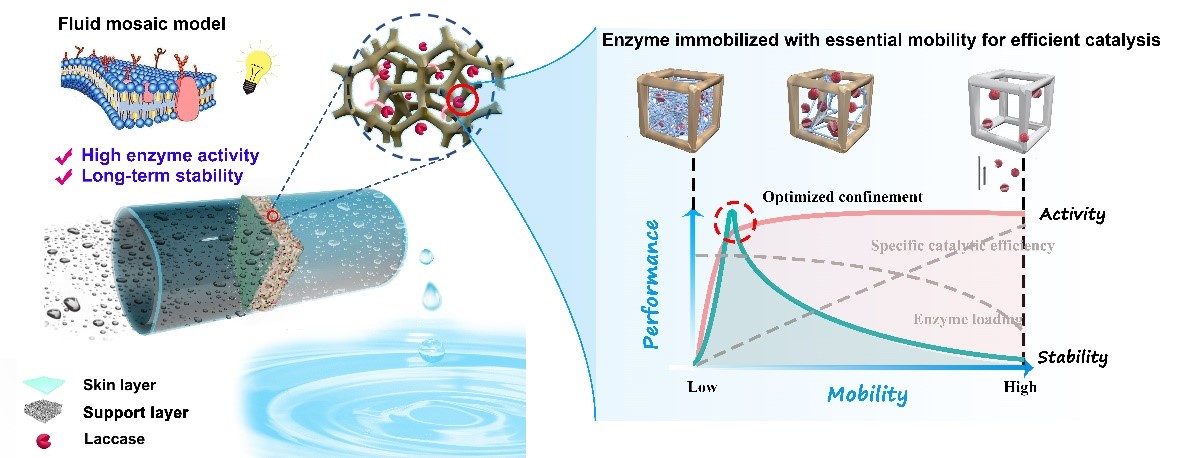
Fig.1 Biocatalytic membrane construction hypothesis inspired by the fluid mosaic model of the cell membrane structure (Image by ZHANG Hao)
Inspired by the fluid mosaic model of the cell membrane structure, a research group led by Prof. WAN Yinhua from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences prepared a novel biocatalytic membrane with high enzyme activity and stability for removal of micropollutants. The study was published in Chemical Engineering Journal on Nov. 28 ( DOI: 10.1016/j.cej.2020.127870 ).
The researchers tuned the confinement strength of the membrane to enzyme, thereby regulating the mobility of the immobilized enzyme via three-dimensional (3D) modification of the support layer of nanofiltration membrane.
Mussel-inspired coating was applied to modify the whole support layer of nanofiltration membrane (termed 3D modification), and laccase was subsequently noncovalently restricted in a modified nanofiltration membrane by reverse filtration.
"Laccase can be stabilized in the 3D modified support layer of the nanofiltration membrane with a uniform distribution, high enzyme loading, and ultra-high storage stability. Moreover, the modified nanofiltration membrane is versatile for different enzyme immobilization," said Prof. WAN.
Even better, this mussel-inspired 3D modification strategy enhanced the confinement strength of the membrane to enzyme with little increment in mass transfer resistance for substrate and products, which effectively delayed the enzyme leakage and at the same time endowed enzyme with a level of mobility for efficient catalysis.
The prepared biocatalytic membrane with optimized confinement strength exhibited high catalytic activity and long-term stability in both seven reuse cycles and 36 h continuous operation for micropollutant removal.
The researchers also proposed a simple protocol to quantify the mobility of the immobilized enzyme, which could precisely reflect the confinement strength of the modified membranes, as well as the catalytic performance of the biocatalytic membranes.
Moreover, the modified membrane was expected to be served as an enzyme storage and controllable sustained-release device for reaction and dosing.
"This work not only offers a versatile platform to immobilize various enzymes and prepare superior biocatalytic membrane," said Prof. LUO Jianquan from IPE, "but also provides guidance to design an optimal confinement environment for enzymes in the membrane, facilitating potential applications of the biocatalytic membrane in enhanced bioconversion, drug delivery, and biosensors at a small scale."
Media Contact:
LI Xiangyu
Public Information Officer
Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-82544826
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号