Fuel cells have attracted much attention due to excellent properties, such as high energy conversion rate, low operating temperature and environmental friendliness, which are considered as the most ideal power supply device for portable electrical appliances and electric vehicles. The bottleneck of fuel cells lies in the sluggish oxygen reduction reaction (ORR) on the cathode. Traditional ORR catalysts are platinum catalysts, which have high cost. In the power generation system of fuel cells, the cost of electric reactor in fuel cells accounts for more than 50% of the total cost, and the cost of platinum catalysts accounts for more than 50% of the electric reactor. Therefore, it is necessary to develop low-cost catalysts to promote large-scale application of fuel cells.
Nitrogen-doped carbon-based catalysts have been confirmed to exhibit good catalytic activity and stability, which can be promising alternatives to noble metals. There are different forms of nitrogen doping configurations, including pyridinic nitrogen, imine nitrogen, pyrrole nitrogen, amino nitrogen, nitrile nitrogen, graphitic nitrogen and oxidized nitrogen. It is generally believed that pyridine nitrogen creates the ORR active site, while other nitrogen doping forms are rarely reported in high-performance ORR catalysts.
Recently, a research team by Prof. WANG Dan from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences (CAS) introduced a new doping form of nitrogen, i.e., sp-hybridized nitrogen (sp-N), into chemically defined sites of ultrathin graphdiyne. The as-prepared sp-N-doped graphdiyne catalyst exhibits excellent comprehensive ORR performance. In addition, the doping site and proportion of sp-N atoms can be well controlled. As shown in Figure 1, a new type of sp-hybridized nitrogen atom has been successfully introduced into specific sites of graphdiyne through the pericyclic reaction. Theoretical and experimental results indicate that, compared with other nitrogen configurations, sp-N atoms make the neighbouring carbon atoms more positively charged, which creates a beneficial chemical environment for O2 adsorption in the ORR. The sp-N-doped 2D carbon catalyst presented an excellent comprehensive ORR performance, even better than that of commercial Pt/C in alkaline solution, and an excellent catalytic activity in acidic solution comparable to that of Pt/C. The optimal sp-N-doped graphdiyne presented catalytic activity superior to that of Pt/C, with an E1/2 of 0.87 V and a Jk of 38.0 mA/cm2 in alkaline solution, as well as better stability and methanol resistance than Pt/C in both alkaline and acidic solutions (Figure 2). The doping strategy to incorporate sp-N atoms into carbon nanomaterials in a controllable way, and the understanding of the doping mechanism, may open new opportunities for site-specific doping of sp-N atoms into other catalysts and thereby broaden the scope of their applications.
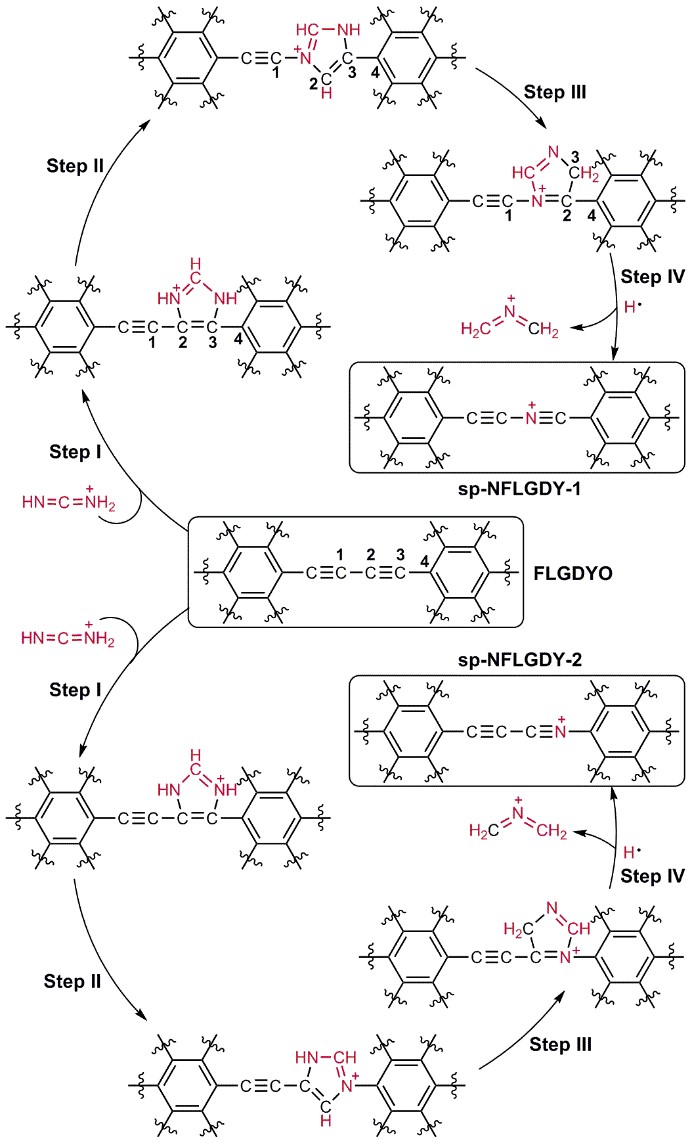
Figure 1 Synthesis of sp-N-doped few-layer graphdiyne. (Image by ZHAO Yasong)
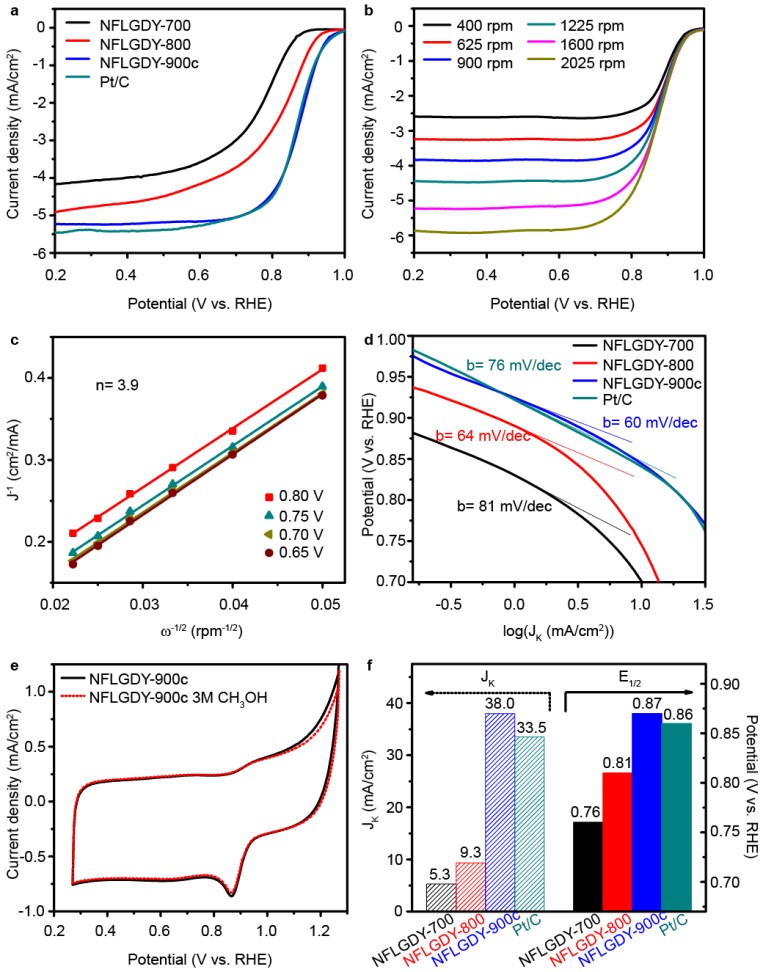
Figure 2 Electrocatalytic ORR activity of N-doped graphidyne and commercial Pt/C in O2-saturated 0.1 M KOH. (Image by ZHAO Yasong)
This work entitled “Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis” has been published in Nature Chemistry (2018, 10, 924).
Media Contact:
LI Xiangyu
International Cooperation Office, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: xiangyuli@ipe.ac.cn
Tel: 86-10-62551358
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号