To combat infectious and chronic diseases, safe and effective adjuvant platforms for prophylactic and therapeutic vaccinations are demanded. Bionic design is regarded as the golden rule in adjuvant design. Previous attempts have focused on the size, shape, charge or compositions of the microbes or diseased cells. However, the fluidity has not been modelled. In fact, antigens on apoptotic and cancerous cells also experience the membrane dynamic curvature and lateral diffusion to increase the contact area and multivalent interactions with antigen-presenting cells (APCs), triggering internalization and subsequent cross-presentation of antigens for robust immune responses. 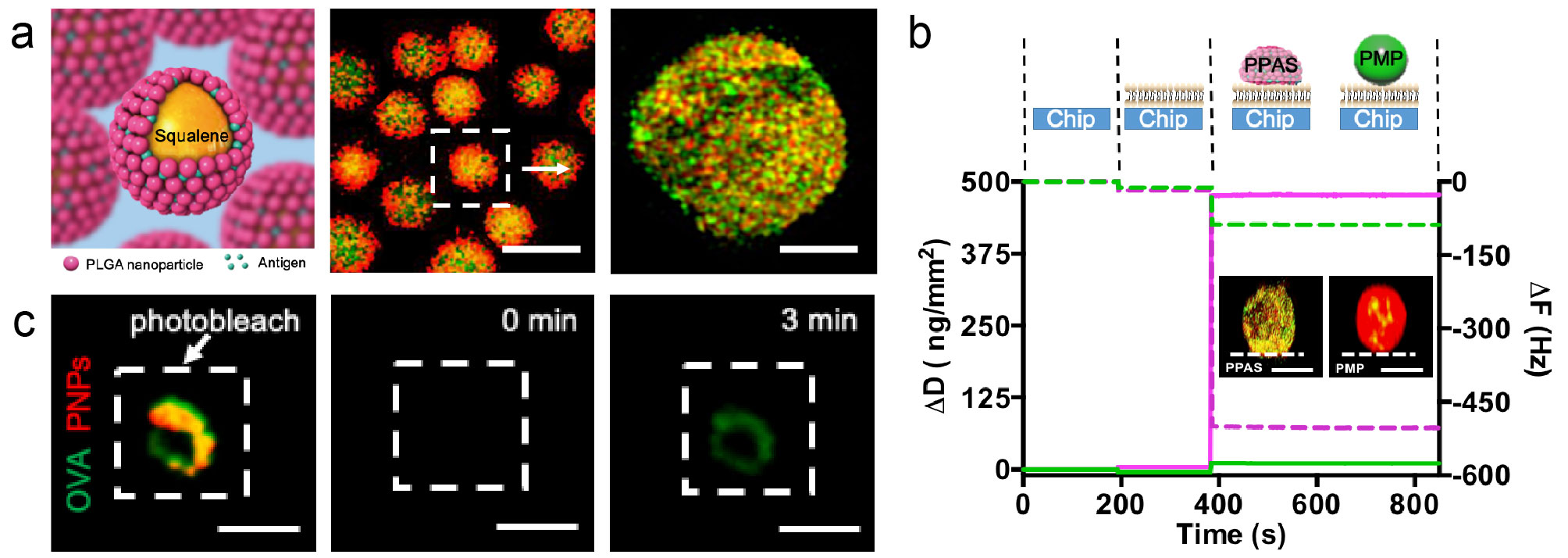
Inspired by this mechanism, research team of Prof. MA Guanghui from State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, re-tailored Pickering emulsion (nanoparticle stabilized emulsion) and heralded as an elastic and biodegradable adjuvant. By creatively combining quartz crystal microbalance with dissipation (QCM-D) and fluorescence recovery after photobleaching (FRAP), Pickering emulsion was unraveled to model after the force-dependent deformation and dynamic fluidity of the natural endocytosis targets during the cellular contact zone (Figure 1). In the absence of surfactants, the raspberry-like morphology of Pickering emulsions offers substantial specific surface area for antigen adsorption and cellular interactions. Thereafter, these special features triggered three-dimensional multivalent interactions with APCs, and subsequently stimulated potent innate and adaptive immune responses (Figure 2a-c). Compared with the clinical relevant adjuvants (Alum, MF59, and AS04), Pickering emulsion droplets were demonstrated with the enhanced immune protections against influenza virus challenge and higher efficiency in both E.G7/OVA and B16/MUC1 anti-tumor immune therapies (Figure 2d-f). The developed Pickering emulsion was validated with high biosafety, stability and bright prospects in clinic applications, which offered an alternative strategy to exploit the force-dependent deformation and lateral mobility of particulate adjuvants for potent vaccinations (Figure 3). Furthermore, preparation technique of uniform biodegradable particle enable the successful construction of this bionic adjuvant with high safety, providing bright application potential.
This work has been entitled “Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination” and published on Nature Materials (DOI: 10.1038/nmat5057, https://www.nature.com/articles/nmat5057). PhD candidate XIA Yufei and Associate professor WU Jie, are the first authors. Prof. MA Guanghui and Prof. SU Zhiguo are the corresponding authors. This study is also contributed by Prof. CAO Xuetao and Prof. WAN Tao from Second Military Medical University, as well as Dr. AN Wenqi and Mr. MA Xiaowei from Hualan Biological Bacterin Co., Ltd. The research is supported by the National Science and Technology Major Project (No. 2014ZX09102045) and National Natural Science Foundation of China project (21576268).
Corresponding author:
Prof. MA Guanghui, Ph.D Principal Investigator
State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences (http://www.bfbm.cn)
Beijing 100190, PR China
Tel: 010-82545001
E-mail: ghma@ipe.ac.cn

Figure 1 PPAS was tailored for adjuvant application. (a) Schematic illustration of the synthesis of DB and biomimetic vaccine construction. Schematic representation, confocal image (Scale bar = 5 μm), and SIM images (Scale bar = 1 μm) of PPAS droplets. PNP and squalene were labeled with Cy5 (red) and DiO (green), respectively. (b) QCM-D analysis on pliability of PPAS and PMP on the membrane-coated chips, with correspondent SIM images. (c) FRAP analysis on the lateral mobility of antigens on the surface of PPAS droplets (Scale bar = 2 ?m). (PNP, PMP and PPAS stand for PLGA nanoparticle, PLGA microparticle and PNP-stabilized Pickering emulsion adjuvant, respectively).

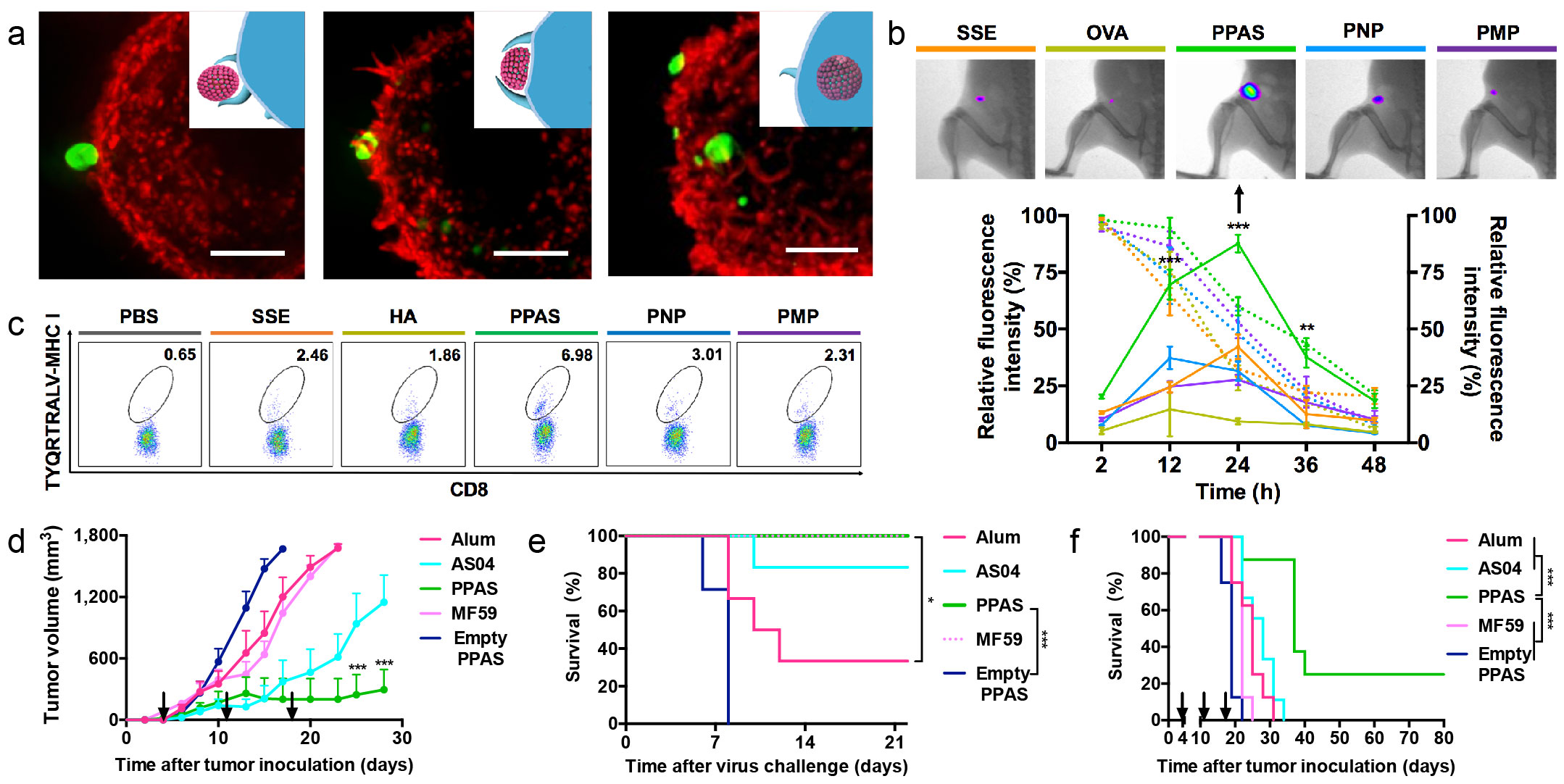
Figure 2 PPAS cultivated APCs to exert systemic immune responses. (a) The process of the phagocytosis of PPAS/OVA complexes by BMDCs. (b) Presence of the migrated antigens in the draining lymph nodes at 24 hours, and quantitative fluorescent intensity of antigens at the injection sites (dotted line) and dLN (solid line). (c) Frequency of HA-specific CTLs in the splenocytes (TYQRTRALV-MHC I+ CD8+ T cells). (d) Evaluations on therapeutic adjuvant activity via tumor volume after vaccination at one-tumor growth curves of the vaccinated mice.

Figure 3 Schematic illustration of the pliability and lateral mobility of Pickering emulsion for enhanced vaccination strategies
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号