Poly(DL-lactic-co-glycolic acid) (PLGA) microspheres have been widely prepared by many methods, including solvent evaporation, solvent extraction and the co-solvent method. However, very few studies have compared the properties of microspheres fabricated by these methods. This is partly because the broad size distribution of the resultant particles severely complicates the analysis and affects the reliability of the comparison.
Recently, the research team led by Prof. MA Guanghui at Institute of Process Engineering (IPE), Chinese Academy of Sciences, explored the exhaustive study on this discrepancy and helped understand the mechanisms of release behavior, degradation and other aspects of microspheres deeply.
Based on Shirasu Porous Glass (SPG) premix membrane emulsification technique developed by the team, uniform-sized exenatide-loaded poly(DL-lactic-co-glycolic acid) (PLGA) microspheres were readily prepared. Exenatide treating type 2 diabetes was chosen as model peptide. The influences on the properties of microspheres fabricated by the aforementioned three methods were intensively investigated, including in vitro release, degradation and pharmacology. It was found that these microspheres presented totally different release behaviors in vitro and in vivo, but exhibited a similar trend of PLGA degradation. Moreover, the internal structural evolution visually demonstrated these release behaviors. The microsphere prepared by solvent evaporation was selected for further examination because of its constant release rate, and explored its pharmacodynamics, histology, etc., in more detail. This microsphere when injected once showed equivalent efficacy to that of twice-daily injections of exenatide with no inflammatory response. This study not only indicated a renewed sense and guidance of microsphere preparation in pharmaceutics, but also fabricated a new formulation for treating type 2 diabetes in clinic.
The results have been published on Acta Biomaterialia, 10 (2014): 4247–4256.
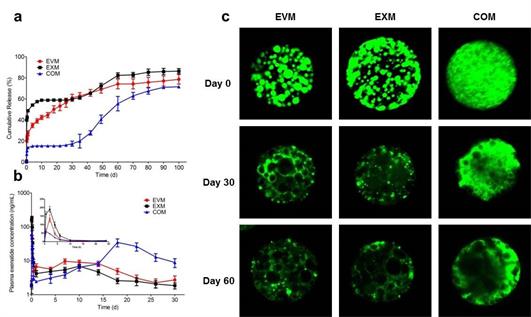
Figure (a) In vitro release profiles, (b) plasma exenatide concentration vs. time profiles of EVM, EXM and COM and (c) CLSM images for inner structural (drug distribution) evolutions of EVM, EXM and COM at different incubation times (day 0, 14, 30 and 60). (By Qi Feng)
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号