Xylan is one of abundant renewable biomass resources for biofuels and industrial chemicals production. The application of extremely thermophilic microorganisms for biomass refinery has been gaining an increasing attention, as process-operating challenge in biomass conversion can be broken. The obligatory anaerobic, extreme thermophilic bacterium Caldicellulosiruptor, grows on a wide variety of carbon sources including pentose, hexose, oligosaccharide, and polysaccharides. The physiological characteristics and highly thermostable carbohydrate-active enzymes of which make it a promising candidate for pentose utilization.
Prof. HAN Yejun's research group at Institute of Process Engineering (IPE), Chinese Academy of Sciences,recently reported novel xylan degradation mechanism of extremely thermophilic bacterium Caldicellulosiruptor.
In the study, the investigators identified the enzymes for thermophilic degradation of methyl-glucuronic acids (MeGlcA) decorated xylan. Two novel intracellular enzymes endo-β-1,4-xylanase (named Coxyn A) and β-1,4-xylosidase (named Coxyl A) encoded by a gene cluster of Caldicellulosiruptor owensensis were heterogeneously expressed, purified and biochemically characterized. Especially, they made an effort to confirm the catalytic model of endo-β-1,4-xylanase and β-1,4-xylosidase versus xylo-oligosaccharides (XOs). The results also indicated the synergism of the two enzymes for xylan backbone hydrolysis.
Moreover, the researchers studied the thermophilic degradation of MeGlcA decorated xylan using glycoside hydrolases (GHs) of Caldicellulosiruptor lactoaceticus. Two novel thermostable xylanolytic intracellular enzymes, GH10 endo-β-1,4-xylanase (named Xyn10A) and GH67 α-glucuronidase (named Agu67A) were obtained and characterized. Experiments showed that beechwood xylan was converted to branched XOs, xylobiose, and xylose by Xyn10A. Agu67A was active on branched XOs with MeGlcA sub-chains, and primarily generated XOs equivalents and MeGlcA. The investigators also observed the synergistic action of Xyn10A and Agu67A with MeGlcA branched XOs and xylan as substrates, both backbone and branched chain of substrates were degraded, and liberated xylose, xylobiose, and MeGlcA.
The two works revealed a novel xylan degradation mode in vitro of extremely thermophilic bacterium which gave a new approach to depolymerize natural polysaccharides at higher temperature. These findings suggested that highly thermostable carbohydrate-active enzymes in extremely thermophilic microorganisms might be ideal candidates for investigating extreme temperature biomass conversion. The characterization of the four GHs provided not only a thermophilic method for natural xylan degradation, but also insight into the mechanism for xylan utilization of Caldicellulosiruptor.
The research articles entitled “Biochemical characterization of two thermostable xylanolytic enzymes encoded by a gene cluster of Caldicellulosiruptor owensensis” and “Insight into glycoside hydrolases for debranched xylan degradation from extremely thermophilic bacterium Caldicellulosiruptor lactoaceticus” have been published online in PloS ONE on the August 15, 2014 and September 3, 2014, respectively. These works were supported by the National High Technology Research and Development Program of China (863 Project, No. 2014AA021905), and 100 Talents Program of Institute of Process Engineering, Chinese Academy of Sciences.
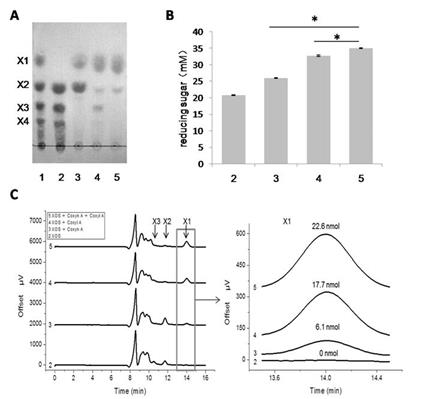
Figure. Hydrolytic mode of xylooligosaccharides by Coxyn A and Coxyl A. (A) Thin-layer chromatography (TLC) assay. (B) Reducing sugar assay of hydrolysis. (C) High Performance Liquid Chromatography assay of products. (Image by Prof. HAN Yejun's group) (Image by IPE)
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号