Influenza, often causing worldwide seasonal epidemics or even pandemics, is a significant cause of morbidity and mortality. Frequent outbreaks in recent years and the increasing number of human infections by highly pathogenic avian influenza (H5N1) virus have accentuated the urgency to develop safe and effective vaccines to prevent a potential pandemic. In view of safety issues, more defined subunit vaccines based on partially purified preparations from the organism or recombinant proteins are more receptive. However, these types of vaccines often show weak immunogenicity due to the lack of an innate immune stimulus. Hence, adjuvants generally are required to facilitate the efficacy of vaccines.
Recently, the research team led by Prof. Guanghui MA at Institute of Process Engineering, Chinese Academy of Sciences, explored the potential of poly(lactic acid)-based microparticles as adjuvant for H5N1 influenza split vaccine.
Poly(lactic acid) is a kind of FDA-approved polymer with good biodegradability and biocompatibility. Microparticles were prepared by oil-in-water emulsion-solvent evaporation method combined with SPG membrane emulsification technique. The adjuvanticity of polymeric microparticles and commercial alum for H5N1 influenza split vaccine was evaluated, and the respective action mode was also investigated. Results showed microparticles-adjuvanted vaccine elicited more potent antigen-specific immune response than alum vaccine. Moreover, compared to alum vaccine, microparticles vaccine elicited less side effects (local inflammation at injection sites). This work indicated that microparticles were promising adjuvant for H5N1 influenza split vaccine.
This work was supported by the 973 Program (Grant No. 2013CB531500), the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KSCX2-EW-R-19), and the 863 Program (Grant No. 2012AA02A406). The results have been published on Pharmaceutical Research. 2014, 31 (4): 1015-1031.
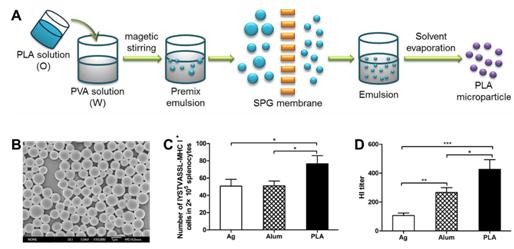
A. Schematic illustration of preparation of microparticles; B. SEM images of microparticles;
C. Cellular immune response (CTL response); D. Humoral immune response (HI titer)(Image by IPE)
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号