Professor WANG Dan from Institute of Process Engineering (IPE) and his group had developed a facile strategy for the fabrication of multi-shelled metal oxide hollow microspheresusing carbonaceous microspheres (CMSs) as the sacrificial templates. By manipulating precursors’ concentration and heating processes, they can easily synthesize large quantities of hollow microspheres with controlled numbers of shells and inter-shell spacings. Experimental data show these architectures can exhibit much better performance than those of their counterparts with single shells when served as gas sensor materials or photoanodes of dye-sensitized solar cells. (Angew. Chem. Int. Ed. 2011, 50, 2738-2741; Adv. Mater. 2012, 24, 1046-1049).
Recently, in order to precisely control the synthesis of multi-shelled Co3O4 hollow microspheres, they further modified their strategy by controlling not only the size and diffusion rate of the hydrated metal cations, but also theabsorption capability of CMSs (Figure 1). Through the methods mentioned above, they can accurately control the number of shells and the interior structures. As a result, multi-shelled Co3O4 hollow microspheres as anode materials for LIBs are obtained with unprecedented high yield and purity. The porous hollow multi-shelled microstructure guarantees more lithium storage sites, a shorter Li-ion diffusion length, and the sufficient void space buffers the volume expansion. Therefore, these multi-shelled Co3O4 microspheres exhibit good rate capacity, excellent cycling performance and ultrahigh specificcapacity (Figure 2). After 30 cycles at a current density of 50 mAg-1, the specific capacity of triple-shelled Co3O4 microspheres remained as high as 1615.8 mAh g-1.
Considering their facile synthesis and the improved performance, the research may open a new avenue for the development of the next generation of LIBs with higher specific capacity, better cycling performance and higher rate capacity. This work has been recently published in Angewandte Chemie-International Edition (Angew. Chem. Int. Ed.2013, DOI: 10.1002/anie.201301622).
This work was supported by the National Natural Science Foundation of China (No. 21031005, 91122014, 21203201, 51172235, 51202248, 21006116, 51272165), and the Foundation for State Key Laboratory of Multiphase Complex Systems (No. MPCS-2012-A-08).
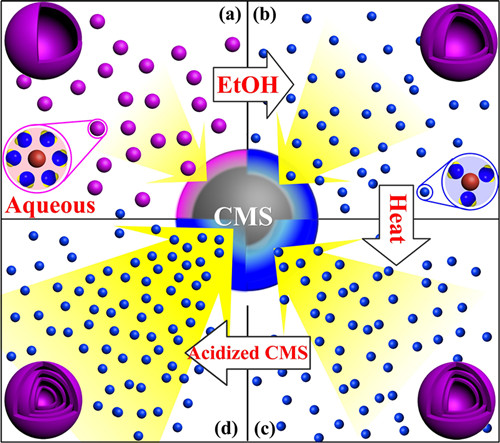
Figure 1.Scheme of the formation mechanism of the multi-shelled Co3O4 hollow microspheres under different adsorption conditions.
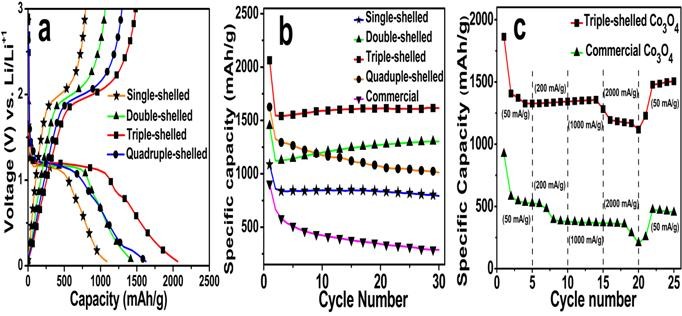
Figure 2.a) First cycle discharge-charge curves of the multi-shelled Co3O4 hollow microspheres at a current density of 50 mA g-1 between 0.05 and 3.0 V. b) Discharge capacities versus cycle number for the prepared Co3O4 hollow microspheres and commercial Co3O4 products at a current density of 50 mA g-1. c) Discharge curves of the triple-shelled Co3O4 hollow microspheres and commercial Co3O4 at different current densities.
Email: danwang@mail.ipe.ac.cn
http://onlinelibrary.wiley.com/doi/10.1002/anie.201301622/suppinfo
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号