Noble metal nanomaterials with customizable internal structures and shell compositions have garnered sustained research interest due to their immense potential for catalysis. Mastery of the structure of nanomaterials enables control of their properties to enhance their performance for a diverse range of applications, such as nanoreactors, drug delivery systems and photocatalysis. Previous methods are either only applicable to a small number of metals or have significant limitations.
Professor Yang Jun with Institute of Process Engineering (IPE) reported a facile general strategy for the synthesis of noble metal nanoparticles with hollow or cage-bell structures based on the inside-out diffusion of Ag in precursor core–shell composite metal nanoparticles.
In the strategy, they first prepared core–shell Ag–M nanoparticles or core–shell–shell MA–Ag–MB nanoparticles in an organic solvent, Ag was then extracted from the core or the inner shell by bis(p-sulfonatophenyl)phenylphosphane dihydrate dipotassium salt (BSPP), which bound strongly with Ag(I)/Ag(0) to allow the complete removal of Ag in 24–48 h, leaving behind an organosol of hollow or cage-bell structured metal nanomaterials. (See the schematic below)The synthesis was carried out at room temperature.
In this report, researchers first demonstrated the preparation of monometallic nanoparticles with a hollow interior, followed by the preparation of hollow nanoparticles with a bimetallic or trimetallic alloy shell, and finally CBS metal nanomaterials with different core and shell compositions.
The hollow and CBS nanoparticles prepared as such had relatively lower densities, which usually translated to a higher surface area than their solid counterparts, the hollow and cage-bell structured metal nanomaterials were especially relevant to catalysis. CBS Pt–Ru nanoparticles displayed excellent methanol tolerance for the cathode reaction of the direct methanol fuel cell (DMFC) as a result of the differential diffusion of methanol and oxygen in the cage-bell structure. The good selectivity was created by the diffusion-limited design, rather than the intrinsic properties of the catalytic metals.
This work was funded by the National Natural Science Foundation of China, 100 Talents Program of the Chinese Academy of Sciences, and the Institute of Bioengineering and Nanotechnology. The paper was published in Journal of American Chemical Society.
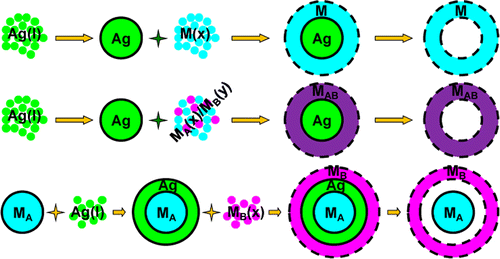
Schematic of the removal of Ag (Image by IPE)
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号