A visible-light-driven (VLD) photocatalyst, Cu2O-CuO/Sr3BiO5.4, was synthesized by an economic and convenient method and achieved good photocatalytic activity, expending the photoactivity to the visible light (VL) region, in particular the sunlight, beyond the traditional titanium dioxide attempts.
Researchers with the Institute of Process Engineering, Chinese Academy of Sciences (IPE-CAS) and Chinese University of Hong Kong co-conducted experiments to prepare photocatalysts by precipitation method using a new acidic system with basic ethylenediamine tetraacetic acid disodium salt (Na2EDTA) as the buffer reagent.
In photocatalytic reactions, 0.1 g L?1 photocatalyst was added into 50 mL diluted E. coli K-12 suspension. The light source was provided by six fluorescent lamps with a total light intensity of 10.5 mW cm?2. The reaction temperature was maintained at 25 °C. The irradiation was under control and different diluted samples were recorded and analyzed. Results showed that part of Cu2O was formed on the surface of Sr3BiO5.4 in situ during the calcinations (see figure), so nanometer-sized Cu2O plays an important role for increasing the photocatalytic activity. 5.4 log inactivation of Escherichia coli K-12 and 27% degradation of naphthalene were respectively achieved in 3 and 2 h under the visible light irradiation provided by fluorescent lamps.
This VLD photocatalyst are expected to have a good performance in potential applications. More studies in this area are being conducted. The paper was published inApplied Surface Science.
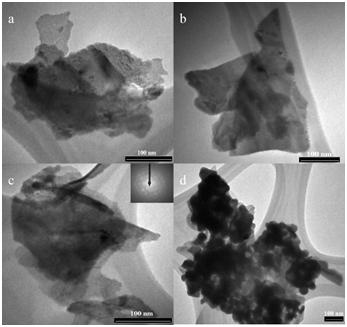
Figure TEM images of Cu2O-CuO/Sr3BiO5,4,(a),(b) and (c) are the images of different particles. The inset of image (c) is the electron diffraction (ED) pattern of this particle. (d) The images of the particles in low resolution.(Image by Wei Zhao)
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号