TLPS composed of organic extractant, water soluble polymer, kosmotropic salt and water is a new separation medium for the treatment multi-component complex system. In the past decades, researchers have applied TLPS for the wastewater treatment, recovery of natural products, separation of bioproducts from the fermentation broth and multi-metal complex systems. However, the mass transfer process remains elusive due to the complexity of TLPS itself.
Researchers from Institute of Process Engineering have discovered the microphase mass transfer process in three-liquid-phase system (TLPS) based on the micellization of block copolymers. Prof. LIU Huizhou and his research group correlated the phase behavior of TLPS with the distribution behavior of three platinum group metals e.g. Pt(IV), Pd(II) and Rh(III) and realized the selective separation between three metals.
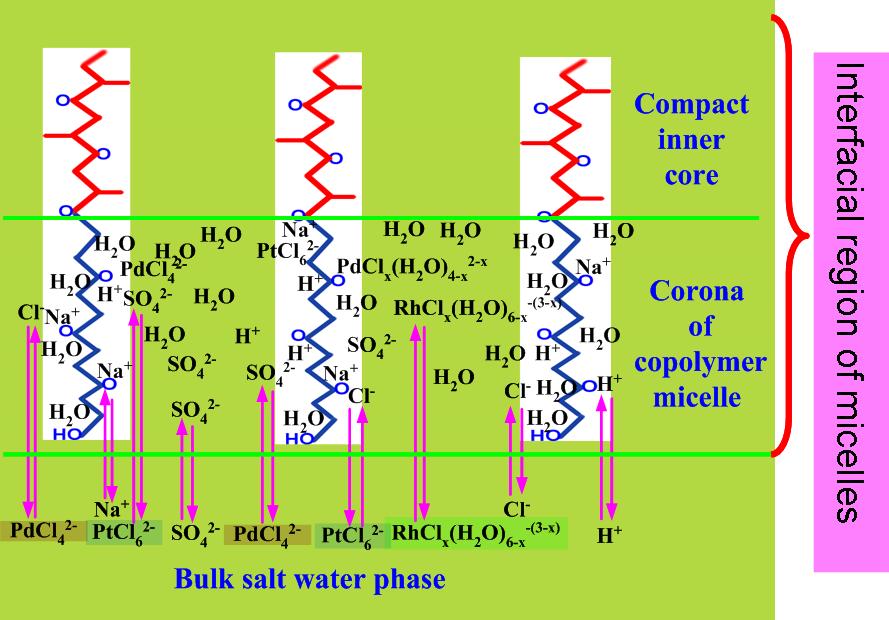
To investigate the mass transfer process, researchers paid much attention to the phase behavior and its interrelationship with the distribution of solutes between three different body phases of TLPS. In this research, the change in apparent phase behavior was expected to be induced by the self-assembly and micellization of EOPO(polyethylene oxide-polypropylene oxide random block copolymer) copolymers. Evolution in microphase interfacial nanostructures was crucial for the selective partition of metal-chloro anions into the EOPO microphases. Micellization of EOPO in aqueous solution was accompanied by the change in microphase structure.
By purposed control over the parameters that affect the micellization of EOPO e.g. Na2SO4, Cl- and H+ concentrations, the selective partition of Pt(IV), Pd(II) and Rh(III) was achieved. TLPS of S201(diisoamyl sulphide)/nonane-EOPO-Na2SO4-H2O was demonstrated an effective separation medium for one-step separation of Pd(II), Pt(IV) and Rh(III) respectively into S201 organic top phase, EOPO polymer middle phase and Na2SO4 aqueous bottom phase through purposed control over the phase behavior.
This work was supported by National Basic Research Programs (973 programs, Nos.2007CB613507, 2007CB714300) and National Natural Science Foundation of China (Nos. 51074150, 21027004) and Innovative Research Group Science Fund (No.20221603). The research results were published in Journal of Colloid and Interface Science (2011, 362(1): 228-234).
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号