Cutting greenhouse-gas emissions and building a low-carbon economy have become significant issues with global concern. At present scientific researchers are focus on the utilization of low carbon energy and reducing carbon-dioxide emissions. CO2 capture and sequestration (CSS) is one of the most practical methods for reducing CO2 emission. However, CO2 capture costs over 75% of CCS method thus technologies of low-cost CO2 capture are expected.
Prof. LI Huiquan and his group from Institute of Process Engineering, Chinese Academy of Sciences developed an effective CO2 adsorbent with high adsorption capacity and selectivity.
This adsorbent, TEPA/MSU-1, was prepared by incorporating tetraethylenepentamine (TEPA) into as-synthesized mesoporous silica MSU-1 with cheap sodium silicate as silica source. In the atmosphere of 10% CO2 mixed with N2, the highest adsorption capacity 3.87 mmol/g was achieved on 50 wt % TEPA loaded at moderate temperature (75℃) and 1atm, and it has a high selectivity of CO2 against N2 (Figure 1). The characterization results suggested that the adsorbent captured CO2 through the formation of alkalammonium carbamate. Furthermore, it can be regenerated completely at 100℃.
CO2 separation from industrial gases such as flue gas of power plant has caused global concern because of the change of climate. Low-cost CO2 separation technologies are essential for the reduction of CO2 emission. Adsorption with solid materials is now considered as a potential alternative of chemical absorption and the research are mainly focus on adsorbent development. This adsorbent might be used for CO2 thermal swing adsorption separation from flue gas of plant power or blast furnace gas without cooling the source gas and easily generated by heating.
This work has been published on Microporous and Mesoporous Materials.
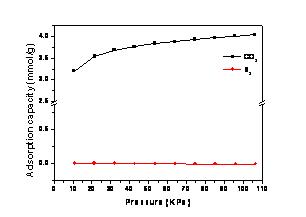
Figure 1 CO2 and N2 isotherms for AM-50 at 75 °C up to 106 kPa
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号