A method to extract gold from quantum dot–gold hybrids or gold-containing alloys could provide an effective route to investigate the influence of gold on the properties of these materials say scientists at the Institute of Process Engineering, Chinese Academy of Sciences(IPE-CAS). The strategy is based on a dissolution–renucleation process which leads to the coalescence of Ag2S and Au nanocrystals at room temperature.
Researchers from IPE demonstrate for the first time an interesting phenomenon occurring in nanoscale materials─a dissolution-renucleation process leading to the coalescence of Ag2S and Au nanocrystals at room temperature in an organic medium. The individual Ag2S and Au nanocrystals in their physical mixture were eventually fused with each other to yield the heterogeneous Ag2S–Au nanocomposites (pictured).
The driving force for the coalescence of Ag2S and Au nanocrystals is due to the equilibration requirement of the Fermi levels between Au and Ag2S, the two different types of particles. Initially, Au and Ag2S nanocrystals undergo Brownian encounters in their physical mixture, and then electrons may tunnel from Au atoms on the surface of Au nanocrystals to their neighbouring Ag2S nanocrystals due to the energy level alignment. Simultaneously, Au ions are emitted from Au nanocrystals into the solution. Finally, these Au ions capture the electrons on the surface of Ag2S nanocrystals, resulting in renucleation of Au on Ag2S nanocrystals.
This unique phenomenon was applied to remove or extract Au from quantum dot–Au hybrids or Au-containing alloys, raising an effective strategy to investigate the influence of Au on the properties of Au-containing hybrids or alloys.
The work has received support from 100 Talents Program of the Chinese Academy of Sciences. The findings have been published in J. Mater. Chem.
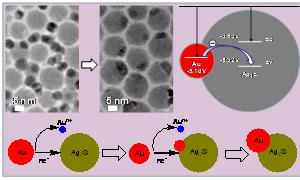
Schematic for the coalescence of Ag2S and Au nanocrystals at room temperature (Images by Professor YANG Jun).
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号