An important frontier in nanocrystal synthesis is the incorporation of different materials within the same nanostructure as a means of increasing functionality. The combination of materials consisting of noble metals and semiconductors within the same nanocrystal is of particular interest. This type of nanostructures combines materials with distinctly different physical and chemical properties to yield a unique hybrid nanosystem with multifunctional capabilities, and tunable or enhanced properties that may not be attainable otherwise.
Researchers from Institute of Process Engineering, Chinese Academy of Sciences and Institute of Bioengineering and Nanotechnology (Singapore) have now developed a semiconductor-noble metal nanocomposite that can improve the performance of direct methanol fuel cells. The nanocomposites were made by depositing various metals, including gold, platinum, palladium, ruthenium, rhodium, and osmium, onto silver sulfide nanocrystals (pictured). In addition to binary nanocomposites, ternary and quaternary composite systems were achieved via the successive deposition of different noble metals on silver sulfide nanocrystals, which makes it possible to control the catalytic properties of the final composite nanomaterials.
The Pt-containing nanocomposites were found to exhibit superior catalytic activity toward room-temperature methanol oxidation, the key reaction in direct methanol fuel cell─an electrochemical device in which the chemical energy stored in a fuel is converted directly into electricity. The researchers explain that the remarkable catalytic performance for methanol oxidation is due to the high surface area of the platinum deposited on the nanocrystals, which provides a larger electrochemically active surface for the methanol oxidation, and also the electronic coupling effect induced by the energy level alignments among the different domains of the nanocomposites. The electron donation from the silver sulfide and gold to platinum leads to a substantial increase in the electron density around the platinum sites, which prevents carbon monoxide, an intermediate during the methanol oxidation, from sticking to the platinum and ‘poisoning’ the catalyst.
Next step, the researchers might potentially design a semiconductor-metal system with enhanced activity toward oxygen reduction, another key reaction in direct methanol fuel cell, whereby the energy level alignment would be favorable for the electron donation from metal domain to the semiconductor domain. The semiconductor–metal nanocomposites could also be of interest as advanced functional materials, and as catalysts for other reactions, such as organic and pharmaceuticals synthesis, environmental catalysis, photocatalysis, and oxidation/combustion reactions.
The work has received support from the Chinese Academy of Sciences and the Institute of Bioengineering and Nanotechnology. The findings have been published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2011, 50, 4637–4643).
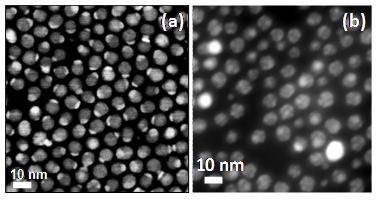
The scanning transmission electron microscopy images of (a) Ag2S-Au and (b) Ag2S-Au-Pt nanocomposites (Images by Professor YANG Jun).
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号