Magnesium chloride is usually generated as byproduct or waste of the potassium fertilizer industry. The valuable magnesium resources cannot be utilized effectively and is discarded back into the sea or saline lakes, causing a serious environmental problem. Therefore, extraction and application of magnesium resources existing in saline brine is growing interesting from an ecological, economic and technological point of view. An attractive way to beneficiate magnesium chloride brine is to prepare high quality MgO. However, the tough problem is how to regenerate or recover the valuable byproducts of NH4Cl.
A new method to recover NH4Cl from NH4Cl-rich aqueous solutions in the magnesia (MgO) production is proposed by researchers from the Key Laboratory of Green Process and Engineering of Institute of Process Engineering. In their paper, the recover process was constructed by modeling the solid-liquid equilibrium (SLE) for the system of NH4Cl-MgCl2-H2O (Fgiure 1 a, b, c) with the Pitzer model embedded in Aspen PlusTM platform. New model parameters were obtained and used to correlate the equilibrium constant for NH4MgCl3·6H2O, which was found to play a key role in the recovery of NH4Cl. The phase-equilibrium diagram (Figure 1 c) generated by modeling is illustrated to identify the process alternatives for recovering NH4Cl. The resulting course of three consecutive crystallizations (Figure 1 d) to recover NH4Cl was finally proved feasible (Figure 1 e). These results are published in AIChE Journal (2011; 57:1595-1606).
The above work was supported by the National Natural Science Foundation of China and the Hundred Talents Program of the CAS.
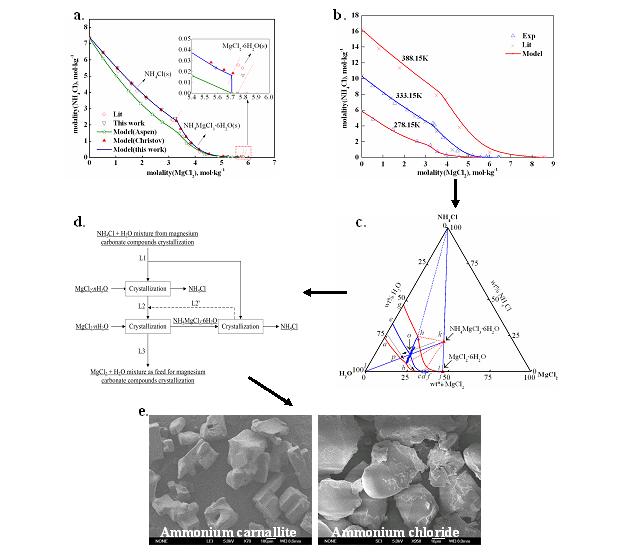
Figure 1 The new recovery process of NH4Cl developed on the basis of solid-liquid equilibrium of NH4Cl-MgCl2-H2O system. a. Solubility at 298.15 K; b. Solubility at 278.15, 333.15, 388.15 K; c. Ternary phase diagram; d. The new recovery process; e. SEM of ammonium carnallite and ammonium chloride.
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号