Researchers from our researchers of Yang Jun team have proposed a strategy for boosting the CO faradaic efficiency in electrocatalytic CO2 reduction reaction (eCO2RR), an attractive option to address serious climate concerns and produce value-added chemical feedstock via coupling with renewable energies. The strategy is promising in producing CO via eCO2RR at ambient conditions.
The study was published in Advanced Functional Materials on Aug. 30.
Among the large variety of products, such as formate, CO, CH4, C2H4, C2H5OH, and CH3OH, converted from eCO2RR, CO is of particular importance.
Unfortunately, although the eCO2RR to CO has the advantage of being carried out at ambient temperature and pressure, it suffers from low faradaic efficiency due to more negative potential than theoretical value, i.e., overpotential, where the hydrogen evolution reaction (HER) is kinetically preferred.
"The key issue addressing the above-mentioned challenge is to design and develop efficient electrocatalysts that are more favorable for catalyzing eCO2RR instead of HER," said Prof. Yang Jun from IPE, corresponding author of the study.
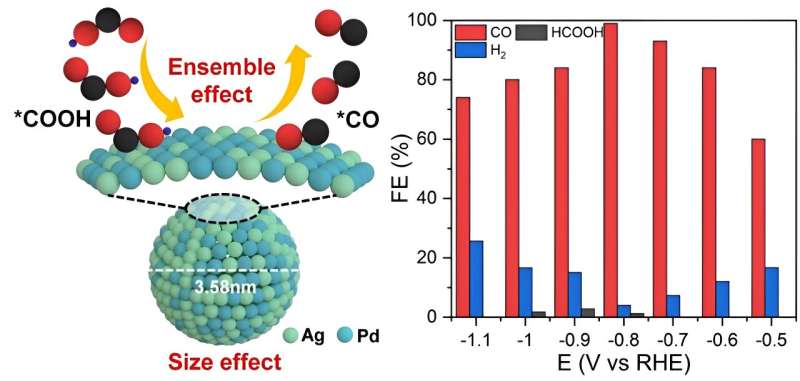
Theoretical calculations validated that the ensemble sites composed of Ag and Pd atoms could promote the eCO2RR by either weakening the CO adsorption or enhancing the COOH adsorption. Based on this, the researchers reported a strategy to produce AgPd alloy nanoparticles with fine sizes for synergizing the ensemble effect and size leverage, achieving high CO faradaic efficiency of up to 98.9% in eCO2RR with satisfactory durability.
"This work highlights the tailoring of active sites via atomic ensembles, which provides a practical method for rationally designing advanced electrocatalysts towards high-efficiency eCO2RR," said Prof. Yang.
More information: Qing Zeng et al, Fine AgPd Nanoalloys Achieving Size and Ensemble Synergy for High‐Efficiency CO2 to CO Electroreduction, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202307444
Journal information: Advanced Functional Materials
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号