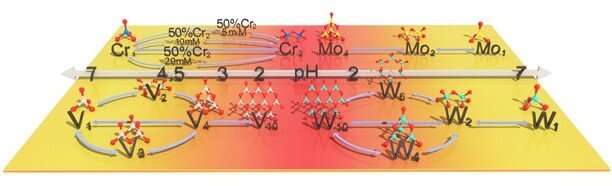
The brief evolution diagram of metal speciation transformation. Credit: IPE
Metals with similar chemical properties are usually extracted together, limiting deep separation for high-purity metal. Different metal species show different activities in solvent extraction. It is necessary to learn about the distribution of aqueous metal species for further extraction.
Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have developed a new strategy to characterize polymeric transition metal species in acidic solution, which may help to separate high-purity metals.
The study was published in Green Chemical Engineering (GreenChE) on June 25.
In the characterization strategy, a high-resolution electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) was employed to measure metal species in aqueous solution, and the corresponding data was analyzed semi-quantitatively.
With this method, the researchers visualized the transformation evolutions of vanadium (V) species, chromium (Cr) species, tungsten (W) species, and molybdenum (Mo) species.
"We can use this facile method to investigate the hydrolysis evolutions of polymeric metal species systematically," said Ning Pengge, a principal investigator at IPE.
The researchers described the formation and polymerization of the four metals (V, Cr, W and Mo) by this method. The obtained species distribution can guide the appropriate reaction conditions for high-purity metal separation. They realized the production of 99.9% high-purity vanadium under 100L/h pilot-scale.
"This work is promising in metal chemical speciation characterization even for Ni, Co, and REE metals," said Ning, "it will guide further development of high-purity metal recovery."
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号