Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences have developed macrophage–tumor chimeric exosomes that co-activate the immune response and tumor microenvironment to support cancer immunotherapy.
Their study was published in Science Translational Medicine on Oct. 13.
Cancer immunotherapies that boost or harness the immune system to battle tumor cells have shown great promise. Most cancer immunotherapies are based on the production of large numbers of immune cells. However, the functions of these immune cells are always compromised by the immunosuppressive microenvironment in solid tumors.
Previous studies have revealed that nano-sized secreted vesicles known as exosomes can act as therapeutic agents and circulating cancer cells have a “homing” capacity to return to main tumor sites.
Inspired by this, the researchers introduced nuclei isolated from tumor cells into activated macrophages, and then prepared biologically reprogrammed macrophage–tumor chimeric exosomes called activated macrophage–tumor cell exosomes (aMT-exos).
“These chimeric exosomes were equipped with various immune components, such as MHC I molecules, costimulatory molecules and immune-activated cytokines. With the aid of their nanosize and tumor-homing molecules, these chimeric exosomes could drain to lymph nodes and accumulate to solid tumors,” said Prof. WEI Wei from IPE.
Inside lymph nodes, the aMT-exos primed T cell activation in both the classical antigen-presenting cell-mediated manner and a unique “direct exosome interaction” manner. Inside tumors, the aMT-exos ameliorated the immunosuppressive tumor microenvironment. Such a co-activation of immune response and tumor microenvironment conferred efficient inhibition of primary tumors, tumor metastases, and postoperative tumor recurrence for personalized immunotherapy.
“Our study illustrated how endogenous cell materials harvested from patients could be used to prepare personalized nano-therapeutics,” said group leader Prof. MA Guanghui. “Considering the safety of utilizing autologous cells and the potent therapeutic effects against various tumors, our chimeric exosome has potential for translation to clinical application.”
A peer reviewer from Science Translational Medicine commented “The generation of hybrid cells to produce chimeric vesicles of this nature is indeed a very novel approach in the field. This is very interesting and exciting research.”
The editor of Science Translational Medicine summarized that “These chimeric exosomes represent a promising strategy for personalized immunotherapy against solid tumors that warrants further clinical exploration.”
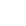 Search
Search




 京公网安备110402500047号
京公网安备110402500047号