Tumor targeting and intratumoral penetration are longstanding issues for cancer therapeutics.
Researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and the University of Chinese Academy of Sciences (UCAS) have developed a new platelet-based formulation which demonstrated potent therapeutic effects against cancer in murine models.
The scientists utilized the aggregation and activation features of the platelets to address issues of tumor targeting and intratumoral penetration. Upon carrying photothermal nanoparticles and immunostimulators, this biomimetic formulation also achieves an efficient combination therapy against multiple types of cancer.
This study was published in Science Advances on March 26.
Recently, photothermal therapy (PTT) has attracted increasing attention. Although promising, efficient delivery of PTT still faces a series of issues. The accumulation of photosensitizers, specifically at tumor sites, and subsequent intratumoral penetration are restricted for most anticancer therapies, because of the cancer’s heterogeneity and the compact extracellular matrix.
As a new type of delivery vector, platelets have shown their capacity to deliver cargo to tumor sites via several mechanisms, suggesting they are reasonable candidates for tumor targeting and intratumoral penetration.
Hyperthermia can induce tumor cells to release antigens. Such a response not only reveals the inherent relationship between the underlying mechanisms of PTT and immunoactivation, but also encourages the combination of PTT and immunotherapy for improved anticancer therapy.
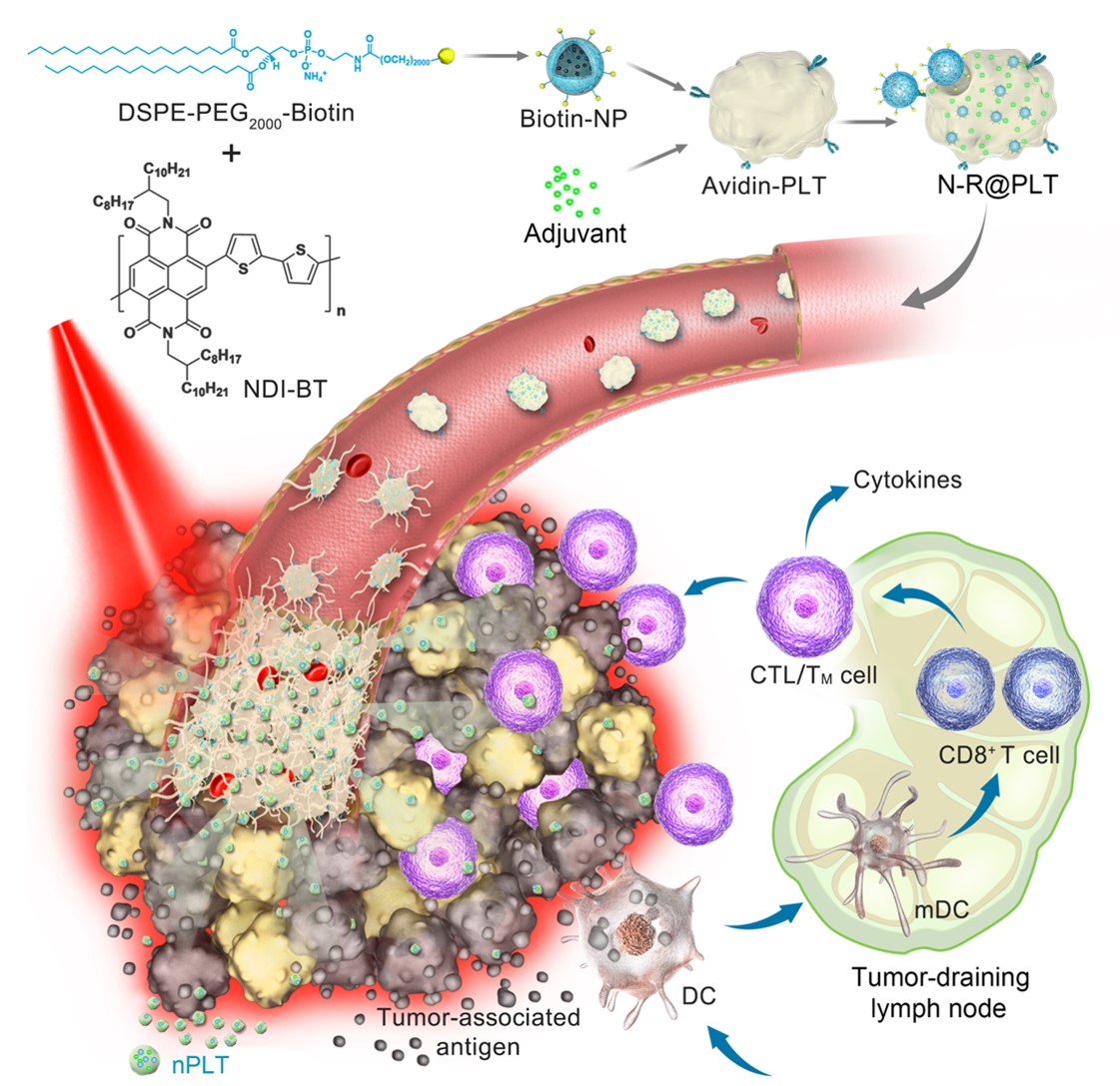
In this new platelet-based formulation, photothermal nanoparticles and immunostimulators were simply, mildly and efficiently integrated into platelets.
“The photothermal conversion efficiency of this novel photothermal nanoparticle reached 69.2%. Thus, low-power near-infrared light (NIR) irradiation can generate enough local hyperthermia,” said Prof. Tian Zhiyuan from UCAS.
The biomimetic platelets worked as circulating sentinels in the bloodstream and had a sensitive response to vascular damage. As a result, a portion of them acted as spearheads to prime adhesion at defective tumor vascular endothelial cells.
After irradiation with low-power NIR, local hyperthermia resulted in acute vascular damage, which subsequently induced an aggregation cascade of reinforced platelets to form a targeting arsenal in situ.
Subsequently, nanosized proplatelets (nPLTs) were further generated upon these activated platelets. “We observed that nPLTs relayed the cargo into deep tumor tissue, expanding the area of attack,” said Prof. Wei Wei from IPE.
Following tumor ablation induced by photothermal therapy, the immunostimulator enhanced the immunogenicity of released tumor-associated antigens, which further induced the body’s immunologic response to attack residual, metastatic and recurrent tumors.
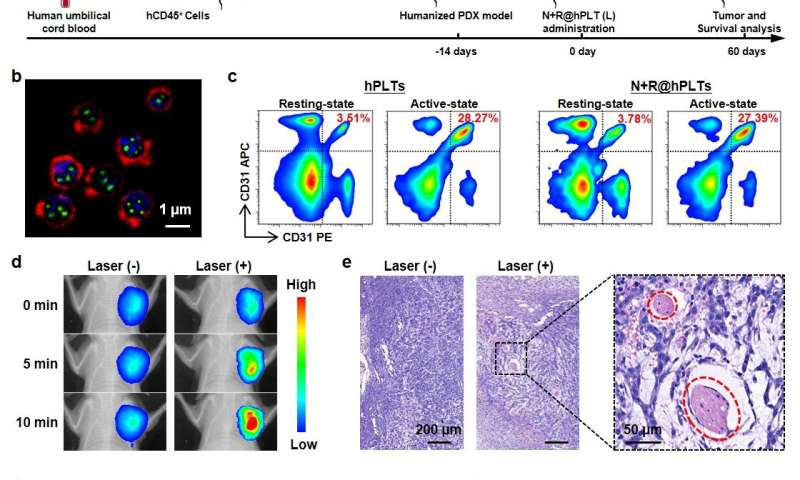
The research demonstrated potent therapeutic effects with low-power NIR irradiation in nine different murine models, and, most notably, a sophisticated model based on human platelets, humanized mice and patient-derived tumor xenografts (PDX).
“These results show great promise for utilization of this novel biomimetic platelet platform in high-performance and combined anticancer therapies,” said Prof. Ma Guanghui from IPE.
A peer reviewer from Science Advances said the study was “well organized and performed.” The reviewer also emphasized that “this system is very effective in tumor therapy and has been shown in different tumor models, and I would very much like to see this work translated into clinical applications.”
 Search
Search




 京公网安备110402500047号
京公网安备110402500047号