Photocatalytic ozonation using graphitic carbon nitride (C3N4) catalysts under visible light (Vis/O3/C3N4) has been proven to be one of the most robust advanced oxidation processes for wastewater treatment. Understanding this process at micro scale and under realistic working conditions is challenging but vital for rational design of catalysts and photocatalytic decontamination systems.
Recently, researchers from the Institute of Process Engineering (IPE), Chinese Academy of Sciences (CAS) and Leibniz-Institut für Katalyse e. V. (LIKAT) have obtained fundamental mechanistic insights into electron transfer and radical transformation by in-situ electron paramagnetic resonance (EPR, see the set-up in Figure 1). They showed that the presence of only 2.1 mol % O3 in the inlet gas stream (in comparison to pure O2) raises the yield of ·OH radicals by a factor of up to 17 and the mineralization rate constant of oxalic acid by a factor of up to 84. They also demonstrated that this was due to a dual role of ozone: (i) O3 traps two to three times more CB-e- than pure O2 and produces ·OH by a robust one-electron-reduction pathway (O3→·O3-→HO3·→·OH) and (ii) O3 can readily take CB-e- back from ·O2- to form ·O3-, thus blocking the inefficient H2O2-mediated three-electron-reduction route which dominated for Vis/O2/C3N4 (O2→·O2-→HO2·→H2O2→·OH, see the mechanism in Figure 2). Their work presents an attractive opportunity to boost the yield of ?OH for water purification by visible-light driven photocatalysis and provides a powerful tool to monitor complex photocatalytic reactions under practical conditions.
The work was financially supported by National Science Fund for Distinguished Young Scholars of China (No. 51425405), Natural Science Foundation of Beijing Municipality (No. 8172043) and the CAS-DAAD fellowship (No. 91637735). The research team includes a group led by Prof. CAO Hongbin from IPECAS and a group led by Prof. Angelika Brückner from LIKAT. More details about this work can be found in the paper published in ACS Catalysis entitled “Fast electron transfer and ?OH formation: Key features for high activity in visible-light-driven ozonation with C3N4 catalysts” (ACS Catal. 2017, 7, 6198-6206).
http://pubs.acs.org/doi/pdf/10.1021/acscatal.7b02180
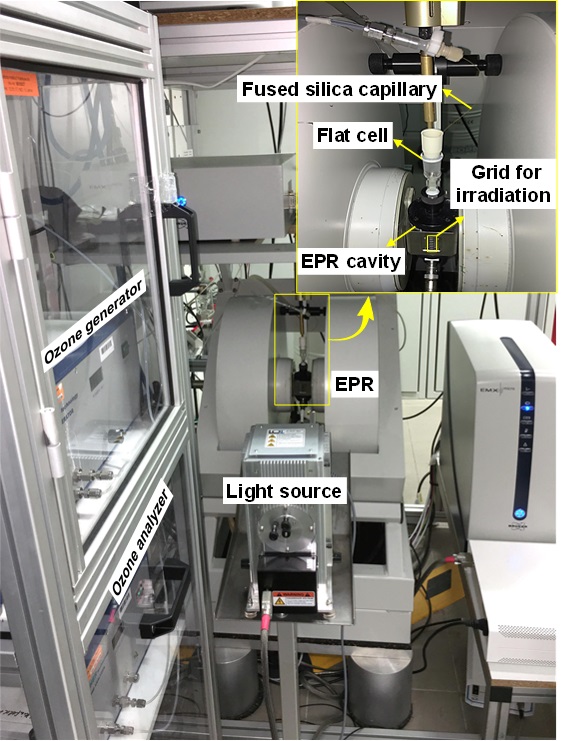
Figure 1. In-situ EPR set-up. (Image by CAO Hongbin et. al.)
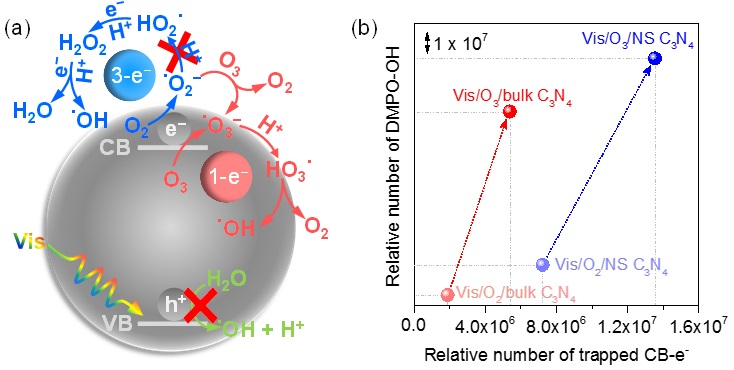
Figure 2. (a) Proposed pathways of ·OH generation by C3N4 photocatalytic ozonation under visible light, (b) Relative number of the DMPO-OH adduct as a function of the number of trapped CB-e-.(Image by CAO Hongbin et. al.)
Media Contact:
YUAN Pei
International Cooperation Office, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: pyuan@ipe.ac.cn
Tel: 86-10-82544882