The water environment, especially the bond-water molecules around the biomolecules (e.g. proteins/peptides), can stabilize the structures/dynamics of proteins, trigger life’s functions and hence inducing various diseases. However, the detecting of the role of water molecules in the process of proteins/peptide self-assembly, the early stage in particular, still have limitations.
Recently, inspired by biology, researchers put forward a strategy, i.e. using ionic liquids (ILs) as a biomimetic medium, to unravel the above issue. Encouragingly, the strategy help researchers gain insight into the key role of trace water in peptide self-assembly. That is because ILs can provide a suitable non-aqueous environment to mediate the content of trace water, enabling the researchers follow the dynamic evolution of peptide self-assembly.
The researchers discovered that the self-assembly of a biologically-derived dipeptide (FF) contains three steps, including nucleation, aggregation of nuclei, and growth for formation of nanotubes (Fig 1). In these steps, the nucleation and growth processes are governed by hydrophobic and hydrogen-bonding synergistic effect.
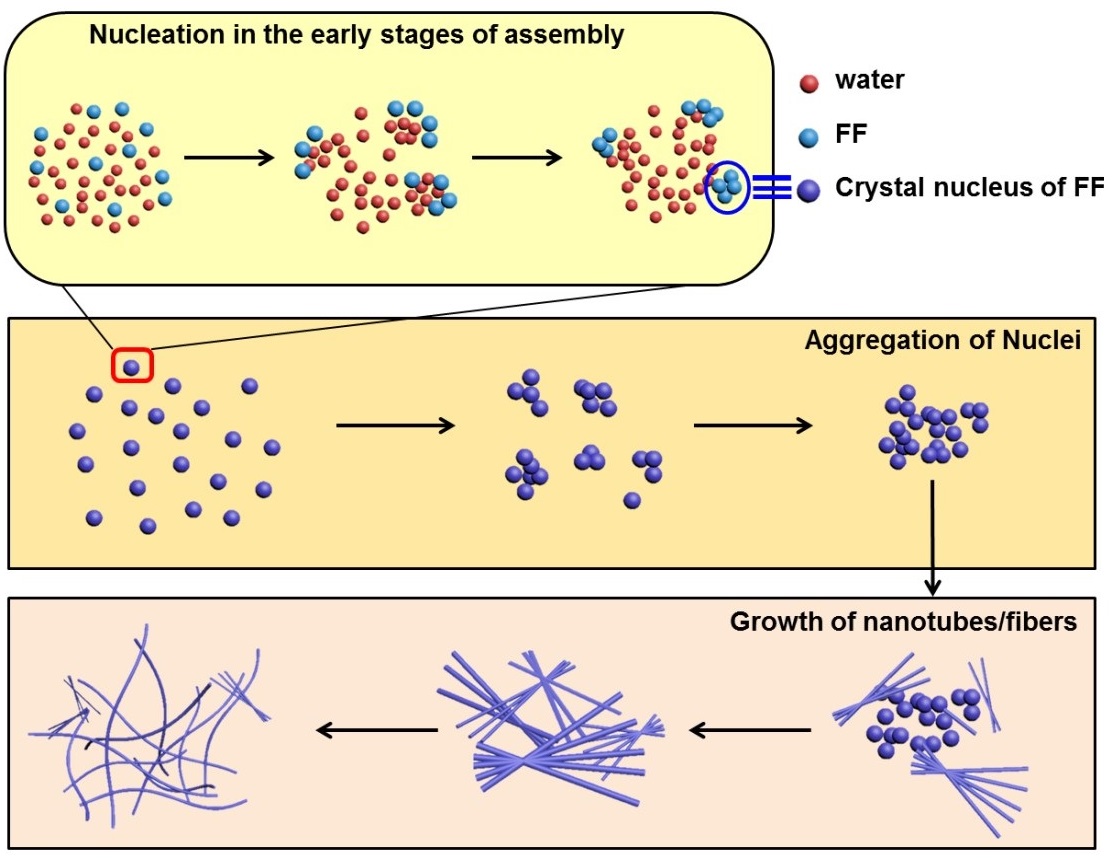
Fig 1. Schematic depiction of the dynamic evolution of FF self-assembly in IL with the increase of water content in IL.(Image by YAN Xuehai et al.)
Moreover, the molecular dynamic simulation results further show that in the early stage of FF nucleation, trace water molecules are essential for the formation of stable noncovalent FF oligomers. As shown in Fig 2, although peptides themselves present a strong spatial correlation or aggregation trend, water molecules present key pioneers to induce the aggregation of FF oligomers.
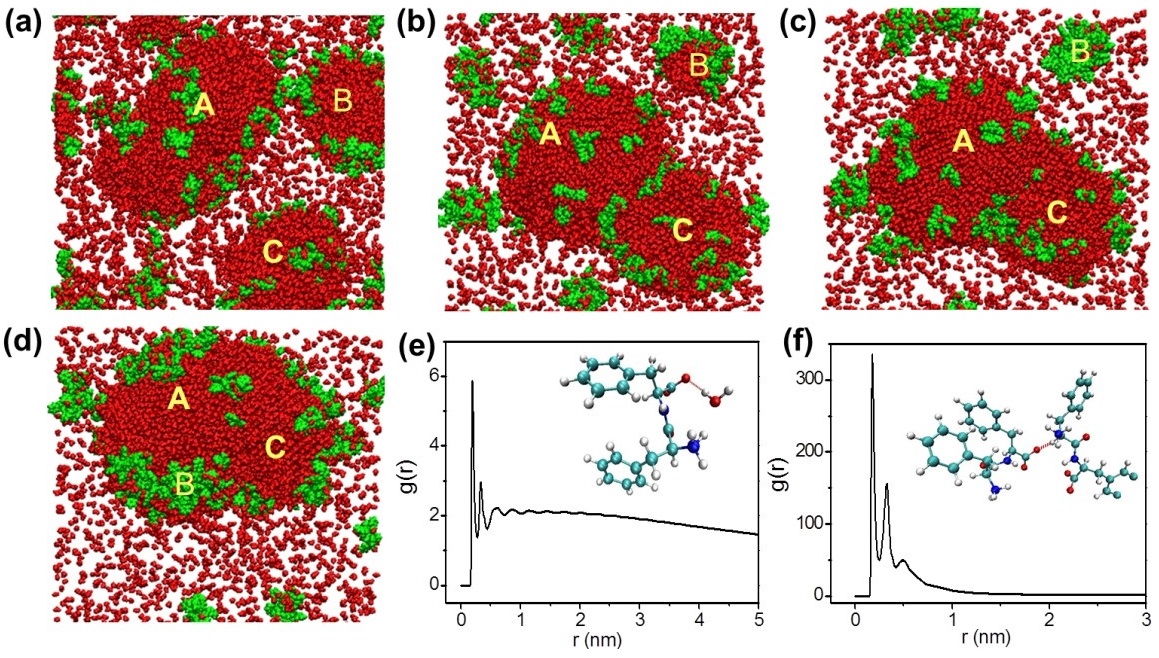
Fig 2. Snapshots of the aggregation process of water molecules and peptide in IL at (a) 90 ns, (b) 470 ns, (c) 560 ns, (d) 3000 ns. Radial distribution functions between (e) peptide and water, (f) peptide and peptide. (Image by YAN Xuehai et al.)
The research team includes a group led by Prof. YAN Xuehai from the Institute of Process Engineering (IPE), Chinese Academy of Sciences (CAS), a group led by ZHANG Suojiang from IPECAS (providing theoretical calculation to this work), and a group led by WANG Yilin from Institute of Chemistry, CAS (providing the ITC measurement to this work). Prof. CHEN Shimou (IPECAS) was thanked for his help for measurement of high-resolution TEM. The research was financially supported by the National Natural Science Foundation of China (Project Nos. 21522307, 21473208, and 91434103), the Talent Fund of the Recruitment Program of Global Youth Experts, the Key Research Program of Frontier Sciences of Chinese Academy of Sciences (Grant No. QYZDB-SSW-JSC034), and the CAS President’s International Fellowship Initiative (Grant No. 2017DE0004 and 2017VEA0023).
Their work entitled “Trace Water as Prominent Factor to Induce Peptide Self-Assembly: Dynamic Evolution and Governing Interactions in Ionic Liquids” has been published in Small, (2017, DOI: 10.1002/smll.201702175).
http://onlinelibrary.wiley.com/doi/10.1002/smll.201702175/full
Media Contact:
YUAN Pei
International Cooperation Office, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: pyuan@ipe.ac.cn
Tel: 86-10-82544882