The researchers from National University of Singapore (NUS), University of California, Riverside (UCR), and Institute of Process Engineering, Chinese Academy of Sciences (IPECAS) have just reported an effective way to achieve the precise control of alloying sites in bimetallic nanoclusters.
One emerging class of metal nanomaterials is thiolate-protected metal nanoclusters (NCs) with particle size below 2 nm, which often features with well-defined molecular formula and structure. Metal NCs in this size regime usually possess strong quantum confinement effects and exhibit some intriguing molecular-like properties, and these properties are inherently (and sensitively) dictated by the size and composition of metal NCs. Therefore, besides size control, composition engineering of metal NCs, such as alloying metal NCs with heteroatoms, provides an efficient way to diversify and tailor the physicochemical properties of metal NCs for practical applications.
In line of alloying metal NCs with heteroatoms, precise control of alloying sites in bimetallic NCs is a central task to realize their structure-/composition-dependent properties. In the past decade, intensive research efforts devoted into cluster chemistry have successfully produced a number of bimetallic and trimetallic NCs at atomic precision, e.g. [Au12@Ag20@Ag12(SR)30]4- and [Ag25(SR)18]- NCs (SR denotes thiolate ligand), leveraging on delicate co-reduction or galvanic replacement reactions. In these successful attempts, Au heteroatoms were allocated in the core region of alloy NCs, most probably due to ubiquitous involvement of the reduction reaction of Au(III)/Au(I) to Au(0). This replacement reaction is thermodynamically favorable since thiolate-protected metal NCs (i.e., Mn(SR)m) typically adopt a core-shell structure of M(0)@M(I)-SR11, where M(0) atoms would preferentially sit in the core of alloy NCs. As a result, Au@Ag NCs (or Au-core/Ag-shell NCs) were generally obtained via the co-reduction or galvanic replacement reactions. However, the same methods could not be used to obtain Ag@Au NCs (or Ag-core/Au-shell NCs), which are thermodynamically less favorable in solution.
Led by Prof. XIE Jianping at NUS, Prof. JIANG De-en at UCR, and Prof. YANG Jun at IPECAS, Dr. YAO Qiaofeng, an assistant professor working with Prof. XIE, and FENG Yan, a doctoral student of Prof. YANG, the researchers present a delicate and well-controlled surface motif exchange (SME) reaction, as illustrated by Fig. 1, which could use the Au(I)-SR complexes in solution to precisely replace Ag(I)-SR protecting motifs on the Ag NC surface, and allocate Au heteroatoms into the protecting shell of alloy NCs. A systematic mass (and tandem mass) spectrometry analysis suggests that the SME reaction is atomically precise displacement of monomeric SR-Ag(I)-SR protecting modules of Ag NPs by the incoming SR-Au(I)-SR modules, giving rise to a core-shell [Ag32@Au12(SR)30]4- NCs. Theoretical calculation suggests that the thermodynamically less favorable core-shell Ag@Au nanostructure is kinetically stabilized by the intermediate Ag20 shell, preventing inward diffusion of the surface Au atoms. The delicate SME reaction opens a door to precisely control the alloying sites in the protecting shell of bimetallic NPs with broad utility.
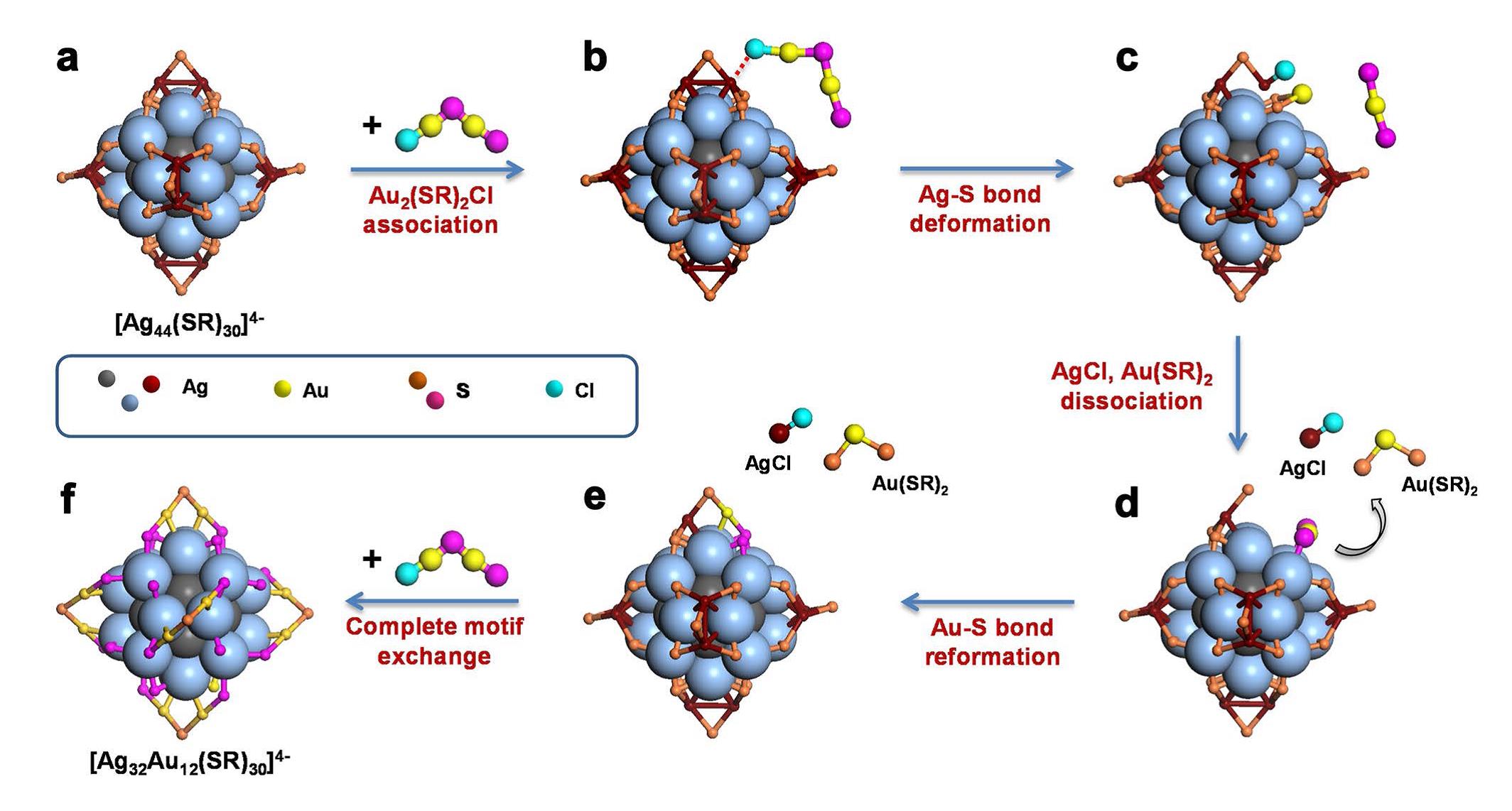
Fig. 1. Schematic illustration of surface motif exchange reaction on Ag44 nanoclusters. For an easy and clear presentation, the Ag atoms in Ag12 inner core and Ag20 external core are shown as grey and light blue large balls, respectively; while the other atoms are shown as small dots. The hydrocarbon tails and carboxylic groups of the protecting ligands are omitted. (Image by YANG Jun et al.)
This study was financially supported by the Ministry of Education (Academic Research Grant R-279-000-481-112), Singapore, and the National Science Foundation of China (Grant No. 21573240), and U. S. Department of Energy (Grant No. DE-AC02-05CH11231). Related finds have been published in Nature Communications with title of "Precise control of alloying sites of bimetallic nanoclusters via surface motif exchange reaction" (Nature Communications 2017, 8, 1115. DOI: 10.1038/s41467-017-01736-5).
https://www.nature.com/articles/s41467-017-01736-5
Media Contact:
YUAN Pei
International Cooperation Office, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China.
E-mail: pyuan@ipe.ac.cn
Tel:86-10-82544882