Global water shortages and pollution greatly limit rapid urbanization and modernization of society. Thus effective water treatment technologies are required for water purification and restoration. For many years, membrane filtration has been regarded as one of the most promising technologies and has been widely used in water treatment due to several unique advantages such as energy saving, small plant footprint, continuous operation and high quality effluent. Nowadays, public concern about the environmental impact of micro-pollutants, including pharmaceuticals and personal care products, steroidal hormones, pesticides, as well as endocrine-disrupting chemicals, has grown due to their high resistance to conventional oxidation methods and negative effects on human health even at very low dosages. This not only provides new opportunities for membrane technology, but also brings some specific challenges. Take the removal of bisphenol A (BPA) as an example, BPA is a harmful endocrine disrupting chemical mainly leaching from plastic products, but it is difficult to remove by membrane filtration because it easily adsorbs and diffuses through the membrane. Thus, relying on the retention ability of a conventional membrane alone would unfortunately never fulfil the stringent emission standards. On this basis the research team reasoned that membranes with multi-functional capacities would be needed in order to achieve a more effective removal of micro-pollutants. Recently, scientists found that adsorbents coupled with membranes (or membrane adsorbers) produced good micro-pollutant removal in short-term operations, nevertheless, after the occurrence of the inevitable “adsorption saturation”, the micro-pollutants’ retention by the membrane were severely decreased, leading to an unsatisfactory permeate quality. The integration of enzymatic catalysis with membrane could help facilitate the micro-pollutants removal. But the current catalytic membrane is based on a micro/ultrafiltration membrane (pore size 1-100 nm), which can’t retain the micro-pollutants, and the short contact time between enzyme and pollutants in flow-through mode limits enzymatic reaction efficiency. Dopamine, an important hormone and neurotransmitter in the human body, can form a self-polymerized coating on various substrates under alkaline conditions, which is inspired by the strong adhesion of mytilus edulis foot. On the other hand, it is well known that biological membranes are able to control substances entering into the cell for metabolism, and inspired by this, researchers designed and made a multifunctional membrane using “reverse filtration” of laccase (a kind of enzyme) solution followed by “dopamine coating” on nanofiltration membrane (pore size 0.4-1 nm) support, which possessed membrane rejection, laccase oxidation and membrane/polydopamine adsorption functions simultaneously. Laccase can oxidize both phenolic and non-phenolic compounds, as well as extremely recalcitrant environmental pollutants within a wide range of pH values, and the final products are usually non-toxic. Rejection of nanofiltration membrane could reduce the enzymatic burden, while the adsorption could enrich the substrate concentration and prolong the residence time which would definitely improve the enzymatic efficiency, and finally the laccase could oxidize the pollutants and broke the “adsorption saturation limits”. These three functions facilitated each other making it a dynamic process, and BPA removal using this novel multifunctional membrane was 84±7.3%, which was superior to the conventional Fenton treatment and was comparable to free laccase coupled with membrane. Their work clearly confirms that the novel multifunctional membrane is promising in tertiary wastewater treatment or household water purifier. 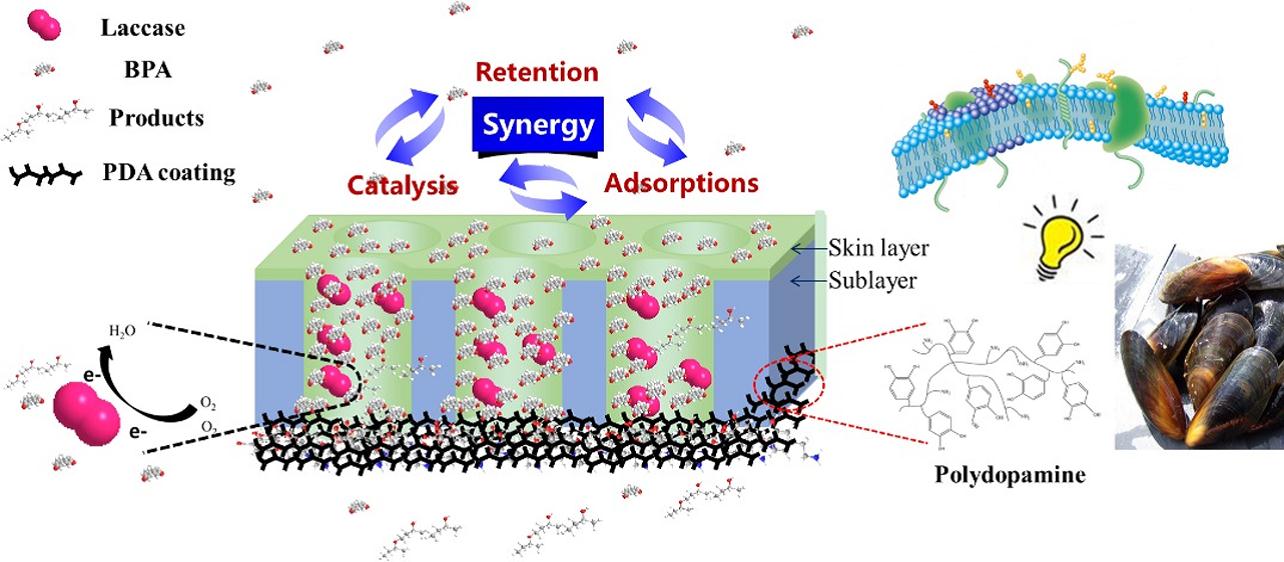
Mechanisms of bio-inspired multifunctional membrane Image by LUO Jianquan The research was supported by National Science Foundation of China (No. 21506229) and “100 Talents Program” of Chinese Academy of Sciences. Their work entitled “Bioinspired Multifunctional Membrane for Aquatic Micropollutants Removal” has been published in in ACS Applied Materials & Interfaces (2016, 8, 30511-30522). http://pubs.acs.org/doi/abs/10.1021/acsami.6b10823 Contact: Prof. WAN Yinhua, Prof. LUO Jianquan State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China E-mail: yhwan@ipe.ac.cn, jqluo@ipe.ac.cn
|