Ionic liquids (ILs) have many favorable physicochemical properties such as low melting points, wide liquid ranges, negligible vapor pressures, high thermal stability, excellent solubility, and tunable hydrophobicity. As a structure-directing force, Hydrogen Bonds (H-bonds) are responsible for ionic pairing, stacking and self-assembling. In particular, H-bonds is considered to be critical in the structure and interaction modes of ILs. In 2007, Prof. Seddon investigated the interactions of ions in the solid state for a series of representative [CnC1im][PF6] salts and found that there are specific directional H-bonds to [PF6]- anions or the formation of interstitial voids. Recently, Prof. Ludwig et al. focused their attention on the characterization of H-bonds in ILs by low-frequency spectroscopy combined with DFT calculations and molecular dynamics (MD) simulations. They found that the H-bonds in imidazolium-based ILs perturb the Coulomb forces to deviate from charge symmetry, finally resulting in decreased viscosities and enhanced fluidization of the ILs. The H-bonds have important effect on the properties of ionic liquid, leading to a decrease in their melting points and viscosities and thus fluidizing them (Figure 1). Up to now, reports on H-bonds in ILs have emerged in large numbers, however, a review on the uniqueness of H-bonds and their roles in reactions has not been presented. In this work, the structural features of H-bonds in common ILs and PILs are discussed based on the results of experiments and calculations, then H-bonds are correlated with the properties of ILs and finally the roles of H-bonds in typical reactions are reviewed. 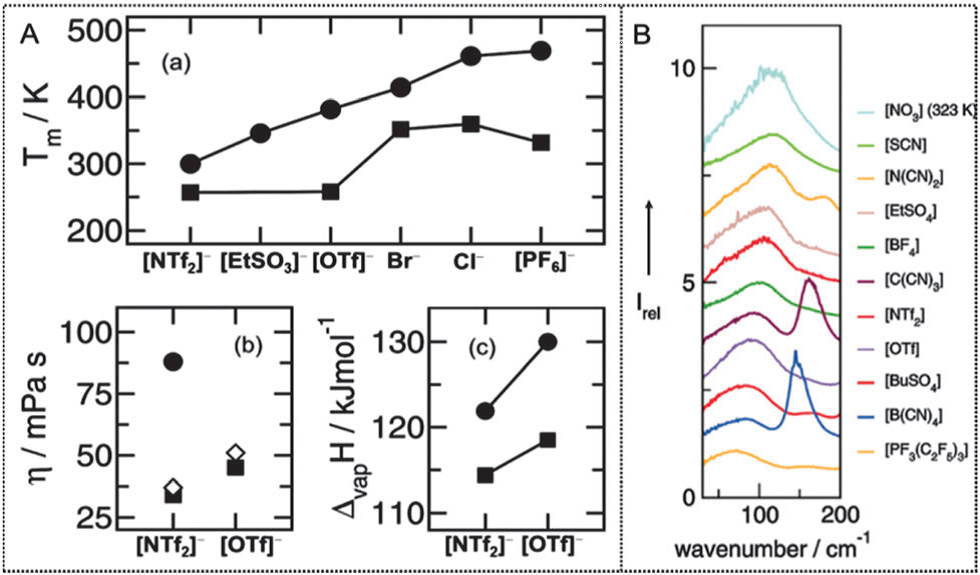
Image by DONG Kun. Figure 1. (A) Anion dependence of Tm, η and ΔvapH values for the imidazolium-based ILs with protonated and C2 methylated cations. (a), Tm of [C2C1im]+ (squares) and [C2C1C1im]+ (circles) containing a series of anions. (b), η of [C2C1im]+ (squares) and [C2C1C1im]+ (circles) with [NTf2]- and [OTf]- anions, and η for methylated ILs at the C5 position (diamonds). (c), ΔvapH of [C4C1im]+ (squares) and [C3C1C1im]+ (circles) with anions [NTf2]- and [OTf]- (B) The measured FIR spectra for 11 imidazolium-based ILs with a series of anions. The research team includes a group led by Prof. ZHANG Suojiang from the State Key Laboratory of Multiphase Complex System of Institute of Process Engineering (IPE), Chinese Academy of Sciences (CAS) and a group led by Prof. WANG Jianji from Henan Normal University. The research was supported by by the International S&T Cooperation Program of China (2014DFA61670) and the National Natural Science Foundation of China (91534116, 91434203, 21276255). Their work entitled “Understanding the hydrogen bonds in ionic liquids and their roles in properties and reactions” has been published in Chem. Commun., (2016, 52, 6744). http://pubs.rsc.org/en/Content/ArticleLanding/2016/CC/C5CC10120D#!divAbstract Contact: Prof. Suojiang Zhang, Director General of IPE, CAS State Key Laboratory of Multiphase Complex System, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: sjzhang@ipe.ac.cn
|