Creating versatile strategies to control the formation of materials has always been a hot issue for both scientific understanding and industrial development. Chemists has ever focused on the traditional layer-wise growth of materials by regulation the atom/molecule-addition process. However, more and more new findings in laboratory and nature have proven that aggregation-base growth by building blocks is also an important way to grow useful materials. In the past decade, researchers devoted great attention to control the aggregation process of building blocks in order to effectively control the shape of materials. Inspired by the continuous reaction tank widely used in chemical engineering, scientists in Institute of Process Engineering, Chinese Academy of Science designed a three-cell reactor which can effectively switch the growth of platinum from the layer-wise growth to aggregation-based growth (Yang Yang, Han Wang, Zhen Ji, Yongsheng Han and Jinghai Li, A Switch from Classic Crystallization to Non-classic Crystallization by Controlling the Diffusion of Chemicals, CrystEngComm 2014, 16 (33), 7633 - 7637). By these three-cell reactors, the two reactants (PtCl62- and reducer) can diffuse into the reaction region at different rates. When the diffusion rate is high (by directly mixing the reactants), the first-stage burst nucleation substantially consumes the precursor, which lowers the concentration below requirement of second-stage nucleation. In this case, the remaining precursor adds to the nuclei by layer-wise growth, which results in defined single crystals. When the diffusion rate is low, the reactants can be continuously fed into the reaction region by slow diffusion. In this case, the later diffusion of reactants into the reaction region could induce second-stage nucleation throughout the crystallization process. As a result, aggregation of nuclei predominate the crystallization when the concentration of nuclei is above a critical level, leading to the formation of dendritic structure of platinum through the aggregation-based growth. This study reveals that regulating the diffusion of reactants can effectively control the crystallization way of materials, which is very helpful for shape-controlled synthesis of materials. And the method proposed in this study is easy to be scaled up for industrial production. Besides, the idea to bring chemical engineering methods into material synthesis is proven to be helpful for developing new frontiers in material synthesis. 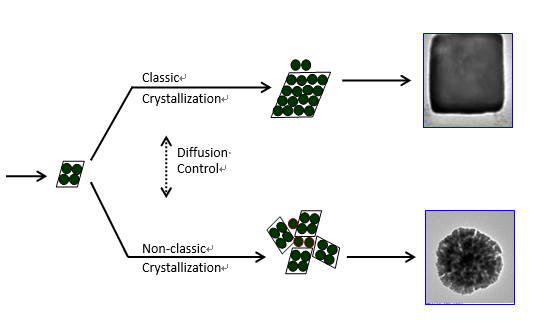
Figure An illustration on classic-crystallization and meso-crystallization. The classic-crystallization refers to an atom-by-atom deposition while the meso-crystallization refers to the assembly of clusters. The switch between these two crystallizations can be realized by controlling the diffusion of reactive ions. (Image by IPE) Key words:diffusion,reactants,formation;materials Contact:Prof. HAN Yongsheng, e-mail: yshan@ipe.ac.cn , Tel: 86-10-82544842
|