CO2 conversion into high-value products is of great strategic significance for utilization of this greenhouse gas. Specifically, the synthesis of cyclic carbonates is a very typical way for efficient transformation of CO2, which has long been a hot issue in catalytic field. However, for commonly-used heterogeneous catalysts, some disadvantages such as scarce active centers for inorganic catalysts and poor thermal stability for organic ones still remain to be overcome for long-term effective industrial application. Thus, it is essential to explore a new heterogeneous catalyst with more active centers and a higher stability. From this point, the research group led by Prof. ZHANG Suojiang at Institute of Process Engineering (IPE), Chinese Academy of Sciences, developed a certain kind of graphitic carbon nitride (g-C3N4) as a heterogeneous catalyst for the synthesis of cyclic carbonates, making up the disadvantages of both inorganic and organic catalysts. Recent reports showed that edge defects are key active sites in defect-containing g-C3N4 for CO2 activation. Enlightened by this, the research group selected urea as precursor for g-C3N4 preparation. The template-free method avoided damage caused by conventional template-removal process, and made more edge defects available. The urea-derived g-C3N4 exhibited higher catalytic activity and stability than the common bulk catalysts for cyclic carbonate synthesis. Furthermore, by adjusting preparation conditions of the catalyst for better performance, the group provides a new method for upgrading catalyst. The results have been published onCatalysis Science & Technology. 2014, 4(6):1556-1562. 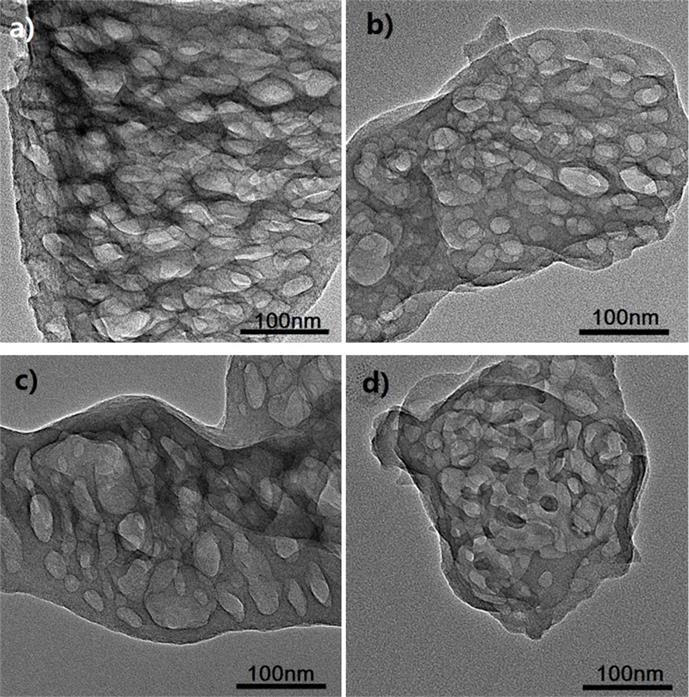 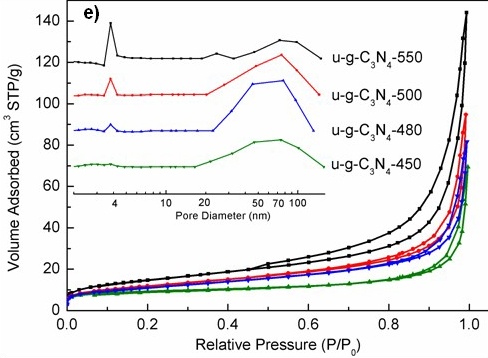
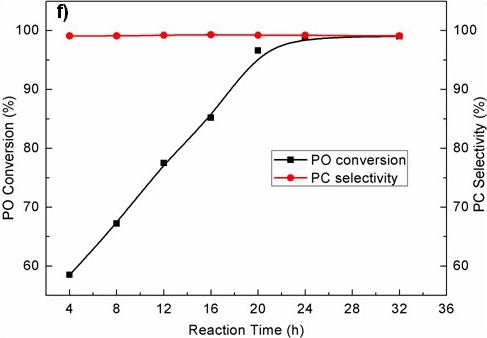 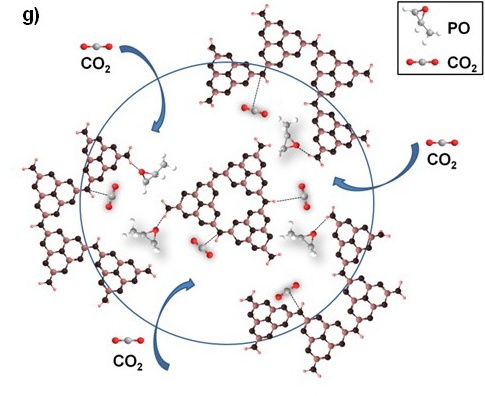
Figure TEM micrographs of graphitic carbon nitrides: a). u-g-C3N4-550, b). u-g-C3N4-500, c). u-g-C3N4-480, d). u-g-C3N4-450; e). N2 adsorption–desorption isotherms of prepared u-g-C3N4; f). The catalytic activity tests of u-g-C3N4-480; g). Illustration for understanding the u-g-C3N4 activation in the reaction.(Image by IPE)
|