Mercury from coal combustion flue gas is present in three forms: elemental mercury (Hg0), oxidized mercury (Hg2+), and particulate-bound mercury (Hgp). Hg2+ is water-soluble and may therefore be effectively captured by wet flue gas desulfurization systems as a co-benefit. Most Hgp can be collected by precipitators. However, Hg0 is the most difficult species to remove because of its high volatility and low aqueous solubility. Hg0 removal from flue gas is becoming increasingly important. Main idea of Hg0 removal is through oxidation into Hg2+, therefore, it is necessary to choose the appropriate catalyst. Recently, the research team led by Prof. ZHU Tingyu at Institute of Process Engineering, Chinese Academy of Sciences, explored the potential of a catalyst for Hg0 oxidation. The catalyst chosen TiO2 as support prepared by an improved impregnation method, and loaded on metal ion, then was investigated for its capability to oxidize Hg0. In this study, 7% CuO/TiO2 was found to be an optimal catalyst with an oxidation efficiency of over 98% at temperatures in the range of 50 to 300 °C. Various characterization methods were used to analyze the catalysts; the results indicated the CuO was well-dispersed on the surface of TiO2 and that Cu2+ was the primary Cu species contributing to Hg0 oxidation. Finally, the mechanism of Hg0 oxidation in HCl/N2-O2 was also investigated. The results have been published on Chemical Engineering Journal 243 (2014) 380–385 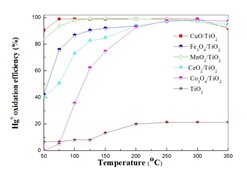  a b  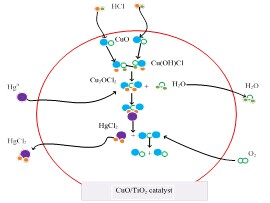
c d Figure a). Results of active components b) Results of CuO concentration c) TEM micrographs of 7% CuO/TiO2 d) The process of Hg0 oxidation
|