Ceria is important in various fields ranging from catalysts, fuel cells, sensors to polishing powders. The performances of ceria vary significantly with different morphologies, sizes, shapes, specific surface areas and exposed crystal phases. Nanoceria with different morphologies, such as nanorods, nanowires, nanopolyhedrons, nanotubes, and nanospheres, have been synthesized by various methods. However, reports of CeO2 hollow nanospheres are few and far between.
Researchers with Institute of Process Engineering (IPE) synthesized CeO2 hollow nanospheres using CeCl3·7H2O and CO(NH2)2 as cerium resource and mineralizer respectively, by a low-cost and environmentally benign one-pot hydrothermal route. Templates, surfactants, or other auxiliaries were not used in the route. This route is sensitive to the synthesis parameters such as reaction temperature, reaction time and the dosages of CeCl3·7H2O and CO(NH2)2.
In their experiment, the morphology and structure of the ceria sample were investigated by SEM and dark-field TEM (Fig. 1). Examination showed that these hollow spheres had a perfect hollow structure and the shells consisted of small nanoparticles. The average diameter for hollow spheres with a shell thickness of 30 nm was about 300 nm.
To further investigate the formation process, the samples obtained after different reaction times were studied in detail by dark-field TEM analysis. Results showed there were two important changes during this transformation: Ce(OH)CO3 solid spheres to CeO2 hollow nanospheres.
The effect of anions on the formation of CeO2 hollow spheres was also investigated. It was found that the presence of Cl− was the crucial factor that induced the formation of the hollow structure.
Besides, CeO2 has been widely used as catalyst. In this work, the ability of ceria as catalyst for CO oxidation was tested. Comparison was made between CeO2 hollow nanospheres and CeO2 nano-octahedrons (Figure 2). The CeO2 hollow nanospheres exhibited a higher catalytic activity on CO oxidation than CeO2 nano-octahedrons due to its high concentration of oxygen vacancy defect and special structure of hollow interior space.
This work was supported financially by the National Natural Science Foundation of China and the National 863 Program. The paper was published in Journal of Nanoparticle Research.
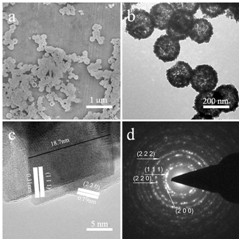
Fig. 1 a SEM image, bTEM image, c HRTEM image, and d SAED pattern of the ceria hollow nanospheres (Image by IPE)
Fig. 2 CO conversion rate in the presence of the as-obtained CeO2 hollow nanospheres (a), and CeO2 nano-octahedrons (b) (Image by IPE)