Researchers with Institute of Process Engineering, Chinese Academy of Sciences (IPE-CAS) successfully prepared CrO2 nanoparticles via oxidation method under high pressure with raw materials of Cr(OH)3·1.1H2O in a closed system. Reaction temperature, time and initial oxygen pressure were 350 °C, 10 min and 6 MPa, respectively.
In the experiment, they first obtained very small Cr(OH)3·1.1H2O particles (Fig.a) with specific surface of 124 m2/g by ball milling. Then under high pressure of oxygen in a close system, nanoparticles of CrO2 (Fig.b)were prepared with the raw material of Cr(OH)3·1.1H2O. The sizes of nanoparticles were from 55 nm to 89 nm (Fig.c), and the magnetization (Ms) of the nanosized CrO2 was 35.522 emu/g.
The phase purity and composition were verified by X-ray technique, and the microstructure was observed by field emission scanning electron microscope. Results showed the influence degree sequence of the four factors was arranged as follows: reaction temperature > mass of H2O > initial pressure of O2 > reaction time. Besides, the sizes of precursor particles were very small and very small particles of the precursor Cr(OH)3·1.1H2O led to the formation of CrO2 nanoparticles under the preparation conditions of CrO2, which researchers proposed as formation mechanism of CrO2 nanoparticles based on the above studies.
CrO2, an excellent magnetic material, has been used in magnetic tapes and disks for several years. With half-metallic characteristics, it may be found application in spin-polarized scanning tunneling microscopy. As for the preparation of CrO2, there have been many ways, such as thermal decomposition method, or sonochemical synthesis, yet no literature reported on oxidation method.
This work was published in Materials Letters.
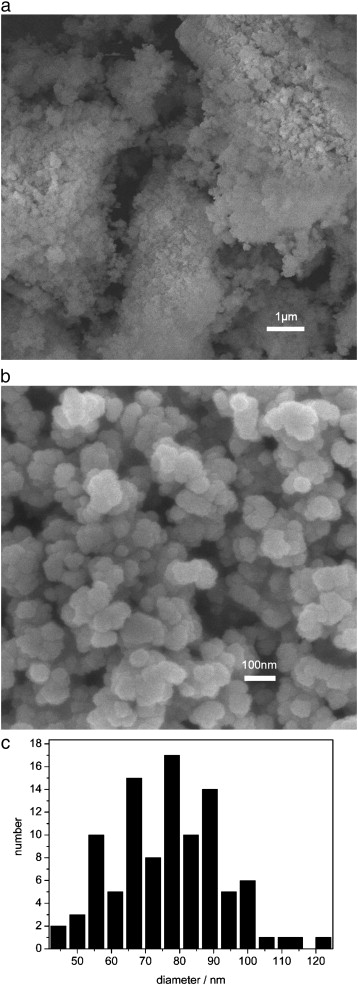
FE-SEM photographs of the precursor Cr(OH)3·1.1H2O and the CrO2 nanoparticles prepared via oxidation process and histogram of CrO2 nanoparticles obtained. (a) Cr(OH)3·1.1H2O, (b) CrO2 nanoparticles (0.2 g Cr(OH)3·1.1H2O, 0.4 g H2O, 6 Mpa, 350 °C, 10 min). (c) Histogram of CrO2 nanoparticles obtained. (Image by IPE)