CeO2, a functional rare earth material, has been widely applied in heterogeneous catalysis field due to its unique oxygen storage/release capacity (OSC), which has been triggered off industrial and academic research interests. It has been reported that the catalytic property of CeO2 is largely influenced by the nature of the exposed crystal planes, which is closely related to its morphology. In addition, oxygen vacancy in the crystal lattice could also promote reducibility of CeO2 materials, even playing an important role. Researchers from Institute of Process Engineering, Chinese Academy of Sciences (IPE) studied the catalytic activities of CeO2 nanofiber and nanocube and compared the catalytic properties of doped-CeO2 nanocube with pure CeO2 nanocube through CO catalytic oxidation as well.
When CeO2 nanofiber and nanocube were chosen to catalyze CO oxidation, the (100) plane of CeO2 for CO catalytic oxidation was more active than (110) and (111) planes and nanocubic CeO2 predominantly exposed (100) plane and nanofiber exposed both (110) and (100) planes. Thus, nanocubic CeO2 had to possess a higher catalytic activity than nanofiber in CO oxidation. However, the result showed that CeO2 nanofiber was more active than CeO2 nanocube. This was because that the former contained more amounts of the oxygen vacancies and larger BET. The concentration of oxygen vacancy for CeO2 nanofiber and nanocube were 5.2×1020/cm3 and 3.5×1020/cm3, respectively.
In order to increase the concentration of oxygen vacancy, a large number of transition and rare-earth metal ions possessing lower valence states were doped into CeO2 since some of cerium atoms can be substituted by those of dopants. The doped CeO2 contains more oxygen vacancies because of charge balance within CeO2 and possesses many novel properties due to the cooperative nature of the cations of the mixed oxides. Among the novel properties, catalytic ability is a focus.
Sm3+ and Gd3+ were used as dopants to dope with CeO2 nanocube. The results indicated that the catalytic activity of Sm-CeO2 nanocube was higher than that of pure CeO2 due to more oxygen vacancy resulted from Sm3+ substituting Ce4+. In contrast, Gd doping exhibited negative effect on CO catalytic oxidation because of the special electron configuration of Gd3+ (4f7) though the formation of more oxygen vacancy (see Figure 1).
This work has been published on ChemPhysChem, 2011.
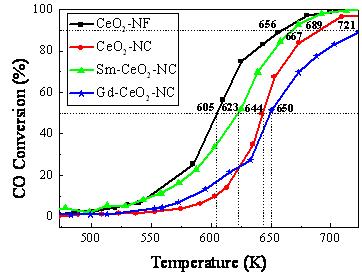
Figure 1 CO conversions over the pure CeO2 and doped CeO2 (Image by WANG Zhen)