"It is not impossible to synthesize noble metal nanomaterials with customizable internal structures and shell compositionsenable control of their properties to enhance their performance for a given application," say scientists at the Institute of Process Engineering, Chinese Academy of Sciences(IPE-CAS), who have developed a general approach for the synthesis of noble metal nanomaterials with hollow or cage-bell structures based on the inside-out diffusion of Ag in core-shell nanoparticles.
Researchers from IPE-CAS discovered for the first time that Ag could diffuse out from the core or the inner shell of Ag-containing single or double shell core-shell metal nanoparticles (see figure). After storage in toluene for several months at room temperature, Ag diffused out from the interior of the core-shell nanoparticles, resulting in a physical mixture composed of isolated Ag nanoparticles and hollow metal nanoparticles.
This interesting diffusion phenomenon could be used to synthesize a wide variety of nanomaterials with hollow or cage-bell structures. The protocol begins with the synthesis of core-shell Ag-M or core-shell-shell MA-Ag-MB nanoparticles in an organic solvent. Ag is then extracted from the core or the inner shell by bis(p-sulfonatophenyl)phenylphosphane, which binds strongly with Ag(I)/Ag(0) to allow the complete removal of Ag in 24~48 hours, leaving behind an organosol of hollow or cage-bell structured metal nanomaterials. Because of their relatively lower densities, which usually translate to a higher surface area than their solid counterparts, the hollow and cage-bell structured metal nanomaterials are especially relevant to catalysis. For example, cage-bell structured Pt-Ru nanoparticles were found to display outstanding methanol tolerance for the cathode reaction of direct methanol fuel cells as a result of the differential diffusion of methanol and oxygen in the cage-bell structure.
The work has received support from 100 Talents Program of the Chinese Academy of Sciences. The findings have been published in Journal of the American Chemical Society (J. Am. Chem. Soc. 2012, DOI: 10.1021/ja302518n).
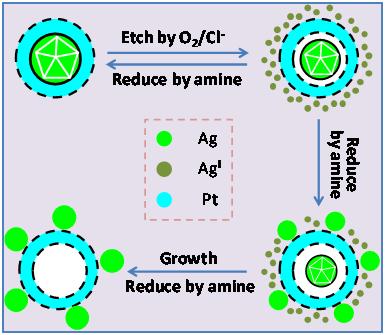
Figure. schematic illustration of the mechanism for the inside-out diffusion of Ag in core-shell Ag-Pt nanoparticles (Images by Professor YANG Jun)